WCOT columns are well suited to mass spectrometry detection and are thus used often. To deposit a film of known thickness, a method consists in filling the column with a solution of stationary phase of known concentration (e.g. 0.2% in ether), so that the desired thickness is obtained after solvent evaporation. This layer can then be cross‐linked by a peroxide or by γ irradiation. The process is similar to the application of paint on a surface that has been pretreated to obtain good adherence.
For packed columns, impregnation or deposit techniques can lead to many different stationary phases from a large selection of low‐volatility organic compounds. On the other hand, for capillary columns, manufacturing constraints require a much more limited selection of compounds. The current phases correspond in principle to three families: polysiloxanes, polyethylene glycols , and ionic liquids . Each category can have many structural variants. For the study of optically active compounds, specific phases are used.
Each of these phases can be used between a minimum temperature, below which concentration equilibria occur too slowly, and a maximum temperature, above which degradation of the polymer occurs. The high limit depends on the film thickness and the nature of the polymer.
Squalaneis used as a reference phase, since it is the only one that is perfectly defined. On the McReynolds scale, squalane has a polarity of zero ( Section 2.10.3). This saturated hydrocarbon (C 30H 62) is derived from squalene, a natural terpene extracted from shark’s liver. On this stationary phase, which can be used between 20 and 120°C (using either deposition or impregnation), the compounds are eluted in increasing order of their boiling points (retention time being inversely proportional to vapour pressure). Various grafted phases based upon polyalkylsiloxanes are also almost apolar.
For capillary columns, polysiloxanes (also known as silicone oils and gums) are the mostly commonly used stationary phases. They are based upon a repetitive backbone that consists of two hydrocarbon chains per silicon atom ( Figure 2.8). Dozens of different compositions of alkyl or aryl chains (methyl or phenyl), onto which can be incorporated further functional groups (e.g. cyanopropyl, trifluropropyl), are available on the market. Monomers combined in various proportions also convey changes in the properties of stationary phases (polarity, stability range). As such, dimethylpolysiloxanes have a very large temperature range, from −50 to 300 / 325°C.
2.6.2 Polyethylene Glycols (PEG)
The best known representative of this family is Carbowax ®. These polar polymers ( M r= 1,500–20,000 – for Carbowax 20M) can be used for deposition, impregnation or as grafted phases, for temperatures not exceeding 260°C, depending on the type ( Figure 2.8).
Column catalogues present optimized phases for special applications: separation of sulfur products, chlorinated pesticides, permanent gases, triglycerides, PAHs, petroleum products or fatty acid esters. Some columns accept high temperatures up to 450°C (e.g. DEXSIL 400 or PETROCOL). The original applications include simulated distillation in the oil industry, replacing conventional distillation, which can take up to 100 hours per analysis.
These stationary phases, developed more recently than the previous ones, are made of di‐ or tri‐cationic salts (ammonium or phosphonium) associated with sulfonate‐ or imide‐type organic anions ( Figure 2.8). For GC, we use viscous ionic liquids with a melting point lower than ambient temperature, due to the fact that these are stable from a heating standpoint and not very sensitive to oxygen. These polar phases, deposited and not grafted on the column surface, are suitable for the analysis of neutral, basic or diverse compounds (fatty acid esters). Elution diagrams are different from those obtained with Carbowax, which makes them useful in two‐dimensional GC. Their near‐zero vapour pressure makes them useful for GC‐Mass Spectrometry.
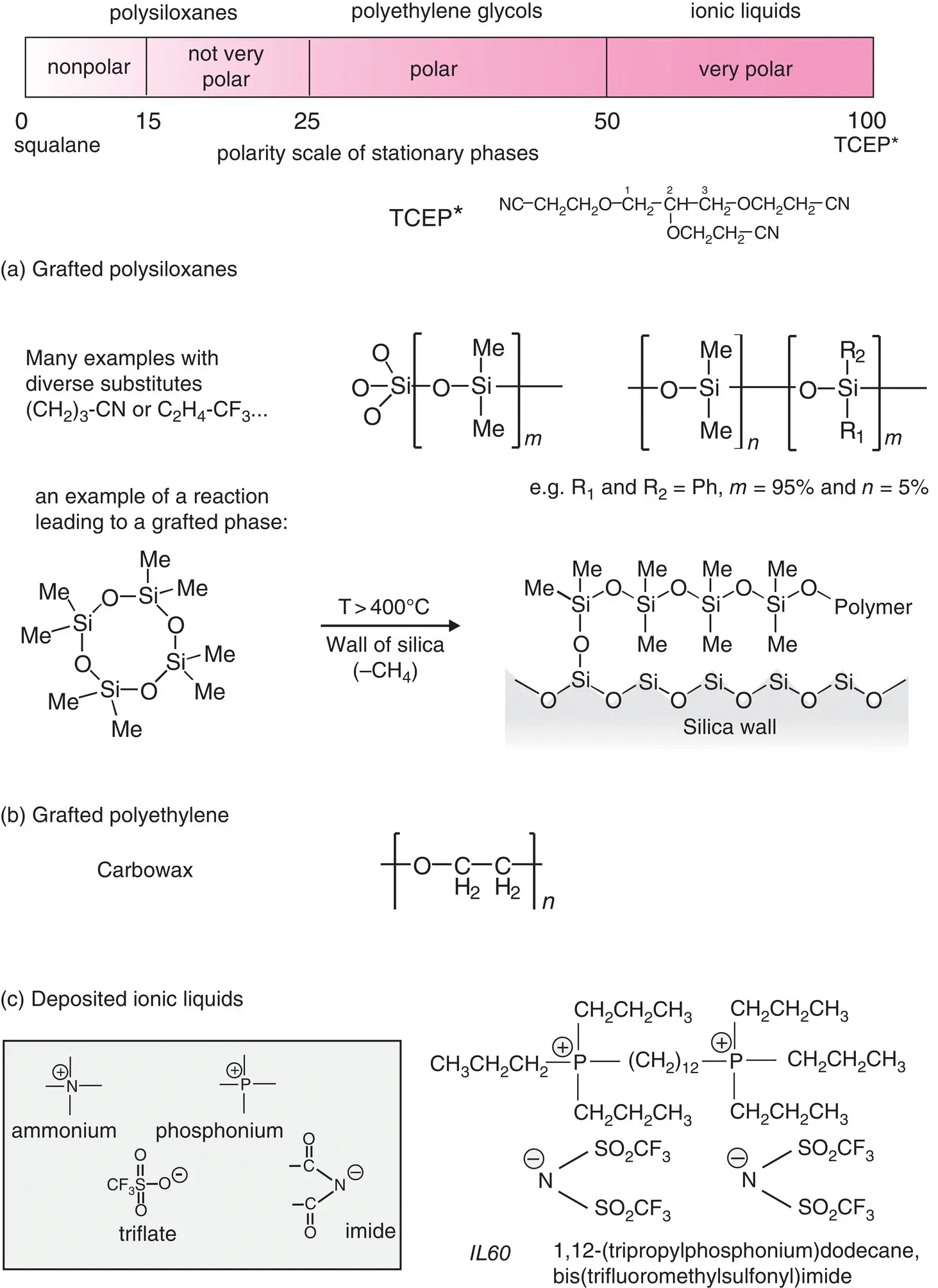
Figure 2.8 Polarity scale of stationary phases in GC and summary of the three types of stationary phase. The polarity scale goes from squalane (polarity 0 by definition) to polarity 100 for TCEP (tricyanoethoxypropane). (a) Polysiloxane structure (silicones), (b) polyethylene glycols . Many phase compositions of this type, used in impregnation or grafting. (c) e.g. a dicationic tetraalkylphosphonium type ionic liquid associated with an imide (here, IL60); max. temperature 300°C.
2.6.4 Chiral Stationary Phases
An organic molecular compound, whose formula demonstrates a centre of asymmetry, leads at our scale, i.e. macroscopic, to a mixture of the two possible enantiomers R and S , in equal quantities or not. Their physical properties are identical, but their behaviour regarding polarized light is what distinguishes them, hence the name of optical activity. In space, these two forms cannot be superimposed. One is the mirror image of the other.
The separation of optical isomers is an important application of chromatography. GC optimizes separations more quickly than other chromatographic techniques (see Chapters 4, 5, and 6). Therefore, it holds a privileged spot in this field, even though the selectivity factor for enantiomer pairs remains close to 1.
Two separations methods exist: direct process and indirect process .
The analyte, supposedly composed of two enantiomers ( R and S ), is chromatographed on a chiral stationary phase composed of a single enantiomer (e.g. R ‐type). Between the analyte enantiomers and the stationary phase, reversible interactions of the R (analyte)/ R (stationary phase) and S (analyte)/ R (stationary phase) are created, with slightly different stabilities. This leads to distinct migration speeds for the two enantiomers. Finally, this leads to two peaks on the chromatogram for this single analyte ( Figure 2.9), whose areas reflect the abundances of the R and S forms, from which the analyte’s optical purity is calculated.
The optical purityof the analyte refers to its enantiomeric excess ( e.e .), calculated from the following relationship, where S Rand S Srefer to the areas of the two enantiomer peaks:
(2.2) 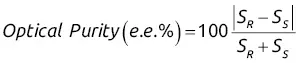
According to this formula, a pure chemical compound present as a racemic mixture will yield two peaks equal in size, each corresponding to an enantiomer. Its optical purity will thus be equal to 0.
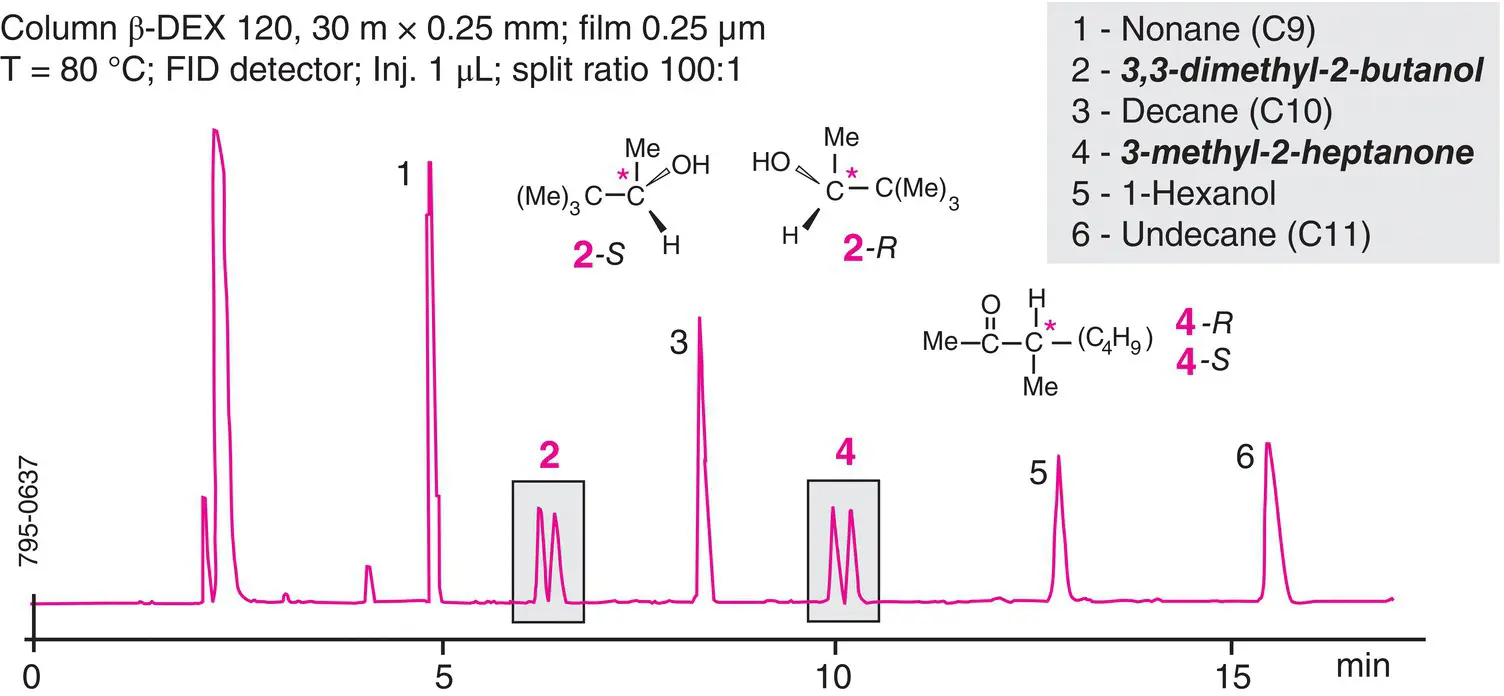
Figure 2.9 Example of a separation with a chiral phase which contains grafted cyclodextrins. The use of a chiral column to separate a racemic mixture of compounds (alcohols 2 and 4). This chromatogram in isothermal mode enables the calculation of retention indexes for the separated compounds.
Читать дальше





![Евгений Матерёв - Музеи… или вдохновляющая музыка The Chemical Brothers [litres самиздат]](/books/437288/evgenij-materev-muzei-ili-vdohnovlyayuchaya-muzyka-th-thumb.webp)







