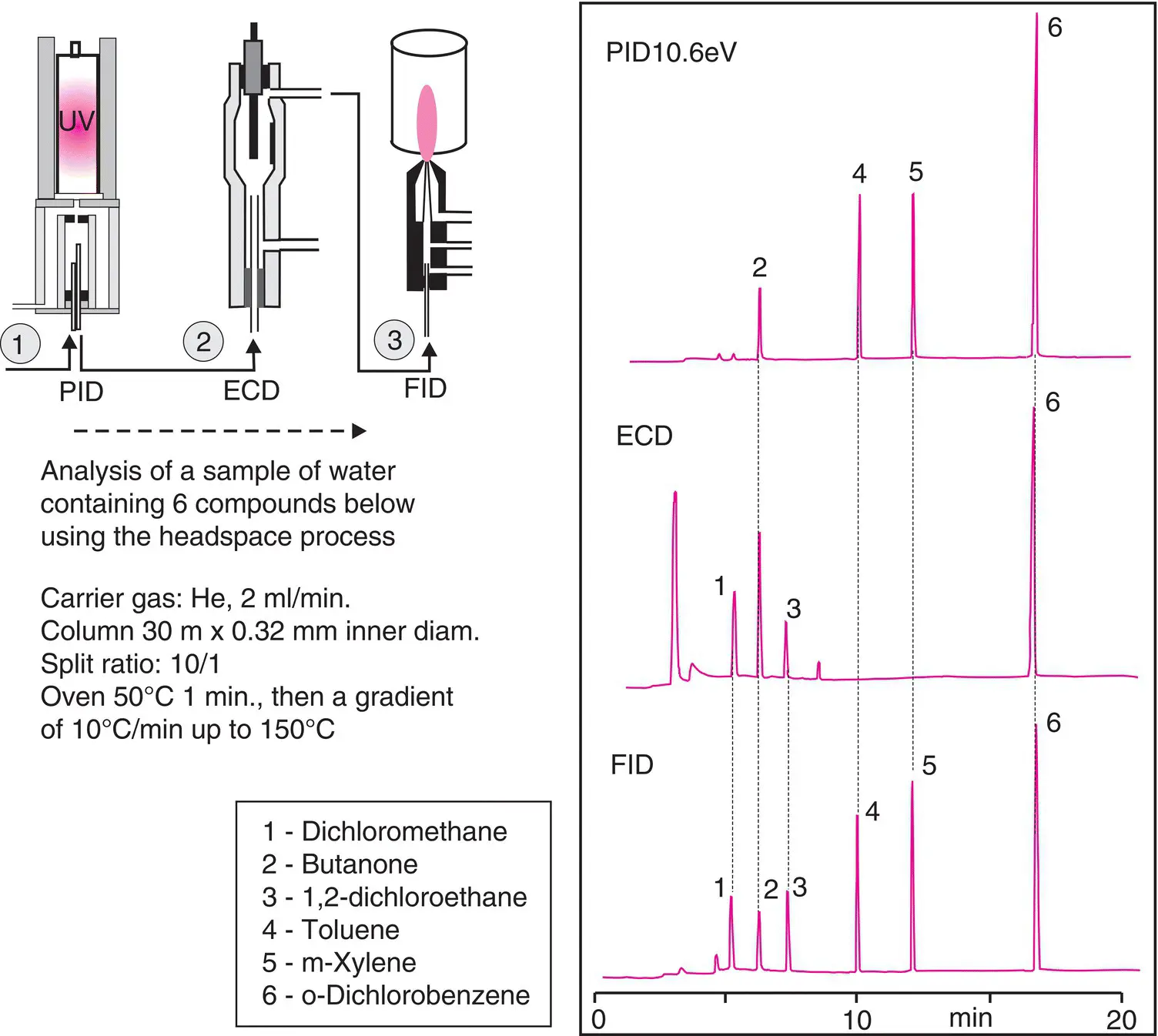
Figure 2.15 Three detectors connected in series. At the outlet of a capillary column, either in series or in parallel and depending upon whether any of them destroy the sample, several detectors can be installed. Here, three chromatograms of an injected mixture as obtained from each detector. Note that the sensitivity varies significantly from one detector to another.
2.8 OPTIMIZATION OF A SEPARATION
If we assume that the optimal stationary phase has been chosen, the length and internal diameter ID of a capillary column as well as its stationary phase film thickness ( d f) must also be taken into account. The conditions for a good separation of the analytes need to be found without increasing the analysis time. From a practical standpoint, in GC, we can only change the temperature and the carrier gas flow rate. In both cases, retention factors k and selectivities α are not much changed. For the carrier gas, we choose a flow rate such that its speed ū is close to the optimal value of the Van Deemter curve. For volatile compounds, we choose a column with a weak phase ratio ( β < 100), hence with a thick film. Inversely, we choose a thin‐film column for less volatile compounds.
However, we must not forget that coupled GC‐MS does not necessarily require greatly optimized separation of chromatographic peaks (sufficient resolution), which is a significant time gain for the chromatographer. Moreover, in a series of analyses, if we can avoid the use of a temperature gradient, we can thus eliminate the time necessary to return to initial conditions in order to conduct the following analysis.
2D GC. Optimization sometimes involves dual chromatography on two different stationary phases (for example, polar and nonpolar) with a single set‐up including a carrier gas inlet shared by the two columns. The first chromatograph is used to isolate a peak, possibly corresponding to an unresolved mixture, which then goes through a second column for another separation.
2.9 FAST CHROMATOGRAPHY AND MICROCHROMATOGRAPHY
2.9.1 ‘Fast’ and ‘Ultra‐Fast’ Chromatography
In general, conventional chromatography is a slow method of analysis. For application reasons (such as control analysis, reaction monitoring, etc.), it may be beneficial to shorten analysis times. To reduce these times, use a shorter column, as retention times are proportional to column length ( Table 2.1). Due to decreased efficiency, the phase ratio β must be increased by choosing a thinner stationary phase film ( ID = 100 μm, film = 0.1 μm, β = 250). Hydrogen must be used as the carrier gas (see Van Deemter curve). The last factor that we can easily regulate is the oven temperature (the higher it is, the shorter the analysis). Be aware, however, of the first peaks appearing, since good separation requires a lower temperature. Therefore, the solution is a temperature gradient. Devices allow for ramps that can go up to 100°C / min. For volatile compounds and with various column designs, covered with a heat‐resistant outer sheath, the temperature can be increased more sharply (200°C / 20 s). As a result, retention times are reduced significantly ( Figure 2.16). This type of fast chromatography , sometimes called high‐speed GC, finds its main use in control analyses.
Table 2.1 Comparison of conventional, fast and ultra‐fast GC set‐ups.
| GC type |
Ramp (°C/min) |
Analysis time (min) |
Peak width (s) |
Column length (m) |
Internal diameter (μm) |
| Conventional |
Conventional oven (1–20) |
~30 |
5–10 |
15–100 |
250–320 |
| Fast chromatography |
Conventional oven (20–100) |
5–10 |
0.5–5 |
5–15 |
100–250 |
| Ultra‐fast chromatography |
Resistive heating (60–1,200) |
~1 |
0.05–0.2 |
2–5 |
50–100 |
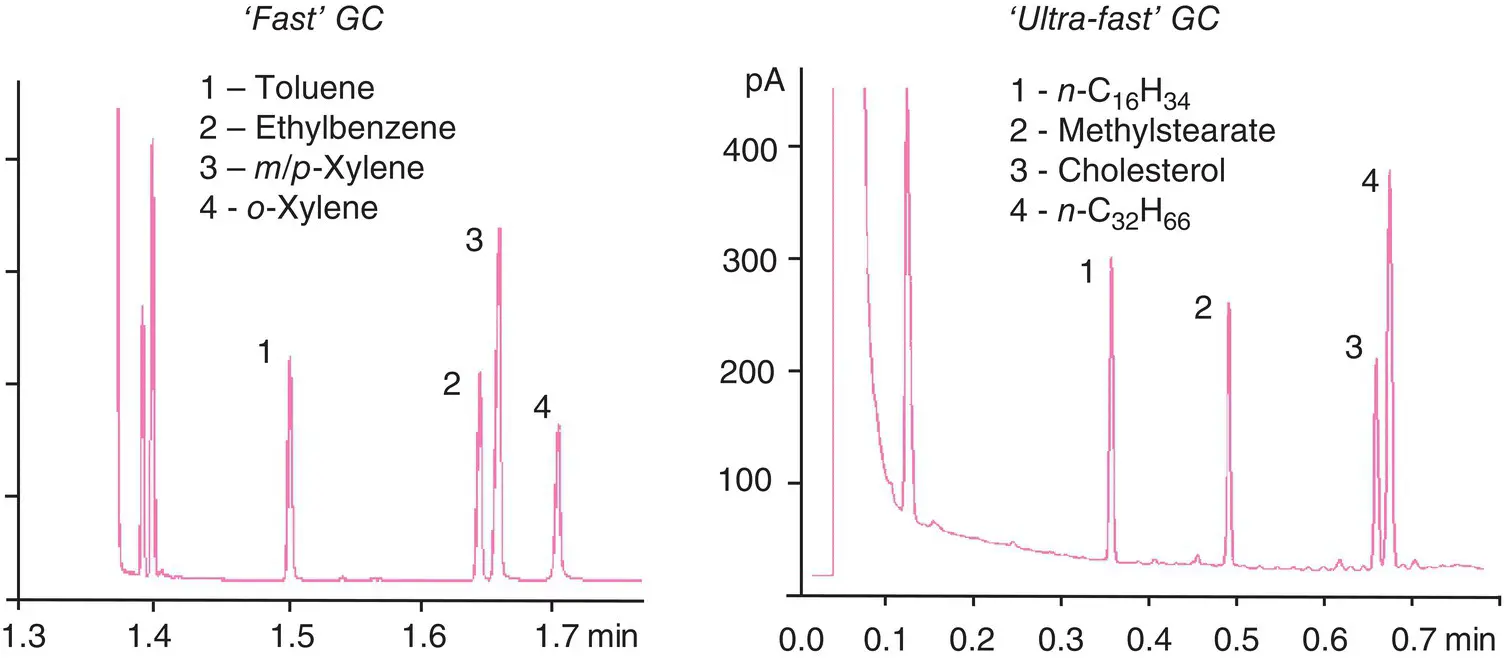
Figure 2.16 ‘Ultra‐fast’ chromatogram. Left, separation of several aromatic compounds (‘fast’ chromatography according to a document from Thermo Electron Corp.); right, example of a chromatogram obtained under ‘ultra‐fast’ chromatography conditions according to a document from Aviv Analytical.
The detector must be able to follow the rapid variations in concentration almost immediately, i.e. at the moment of each analyte’s elution. For detection by mass spectrometry, there is good reason to be attentive to the sweep speed of the m / z ratio; a slow sequential sweep may lead to a situation in which the concentration in the ionization chamber is not the same from one end of the recording to the other. TOF‐MS (Time‐of‐Flight Mass Spectrometry) does not suffer from this drawback.
2.9.2 Micro Gas Chromatography
In parallel with laboratory equipment, portable devices (μ‐GC) have been developed for fast analyses in the field ( Figure 2.1). These devices, sometimes referred to as ‘noses,’ must be light and small, despite a carrier gas reserve enabling their stand‐alone use. The detector used most often is the katharometer with its detection and quantification limits, or more specific variants (μ‐katharometer). The flame ionization detector, requiring an additional gas source, or the mass detector would make heavier and more complex devices. These portable devices may include several analytical modules, operating in parallel for multiple, simultaneous analyses. For each module, simply choose a column with a different polarity and different temperature conditions. In fact, each column is covered with a metal envelope supplied with an electrical current, which enables its temperature programming.
2.10 RETENTION INDEXES AND STATIONARY PHASE CONSTANTS
These parameters have been developed to pursue at least three objectives:
To identify a compound by a more general characteristic than its retention time under predefined conditions. As a result, a system of retention indexes has been developed; it is an efficient and cheap means by which to avoid certain identification errors.
To follow the evolution of a column’s performance over time.
To classify all stationary phases in order to simplify the choice of the column best adapted to a particular kind of separation problem. The polarity or chemical nature of a stationary phase does not allow prediction of which column will be optimal for a given separation. For this, the behaviour of stationary phases with respect to several reference compounds should be examined, in order to determine stationary phase constants.
2.10.1 Kovats Straight‐Line Relationship
Retention indexes are determined using the following general principle. When a mixture containing compounds belonging to a homologous series of n ‐alkanes is injected onto a column maintained in isothermal mode, the resulting chromatogram is such that the logarithm of the adjusted retention times t′ R(n)increases linearly with the number n of carbon atoms present in the corresponding n ‐alkane ( Figure 2.17).
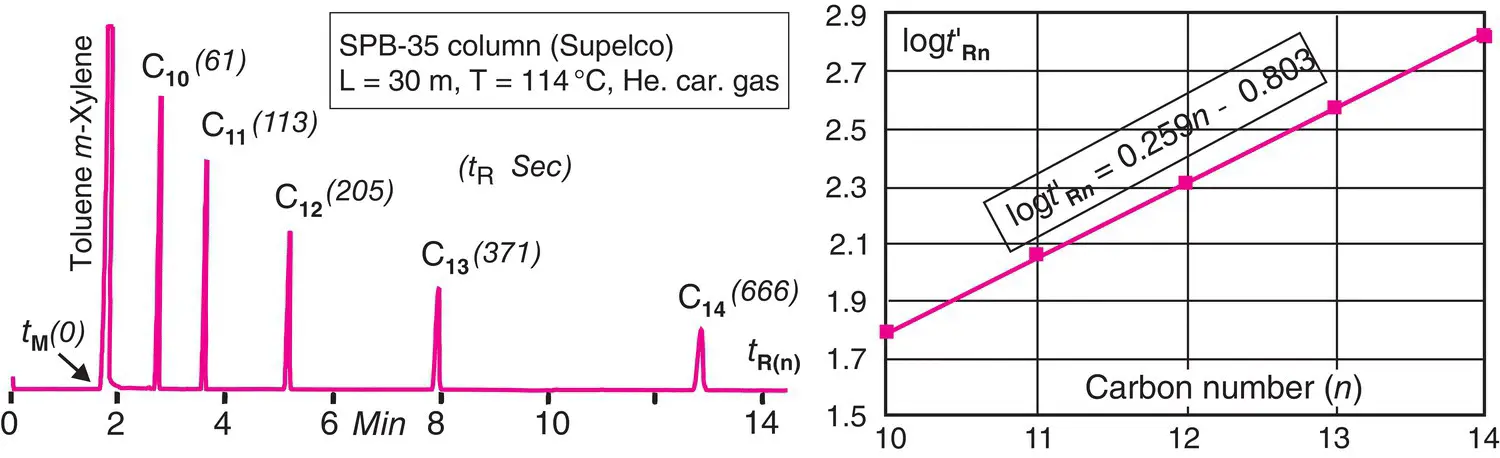
Figure 2.17 Kovats straight line graph. Left, isothermal chromatogram for a series of five n ‐alkanes ( C 10− C 14). Right, Kovats’ relationship for the chosen stationary phase and for the predetermined analytical conditions.
Читать дальше






![Евгений Матерёв - Музеи… или вдохновляющая музыка The Chemical Brothers [litres самиздат]](/books/437288/evgenij-materev-muzei-ili-vdohnovlyayuchaya-muzyka-th-thumb.webp)







