Its operating principle is based on the thermal conductivity of gas mixtures as a function of their composition. The main part of this detector is the katharometer , a thermostatted metal unit that is brought to a temperature slightly higher than that of the column and which includes thermistors located in tiny cavities. In the given example, the katharometer includes four thermistors, placed two‐by‐two and fed as indicated either with carrier gas sourced upstream from the injector or with the mobile phase downstream from the column. When a solute elutes, the conductivity of the mixture (carrier gas + compound) decreases with respect to that of the carrier gas alone. The thermal equilibrium is disrupted and this results in a variation in the resistance of one of the filaments, which is proportional to the concentration of the compound in the carrier gas. The dynamic range of this detector extends over some six orders of magnitude, and while its sensitivity is quite average (from ng to mg), it is being used more frequently thanks to the rise of micro‐GC (see Section 2.9.2).
Mass spectrometry detector (MSD)
For several years now, the rise in mass spectrometry has favoured the use of the coupled GC/MS technique, which consists in connecting the chromatograph to a mass detector (a low‐resolution mass spectrometer, see Chapter 16for more details). Its use as a universal detector for capillary GC has the advantage of leading to a fragmentation spectrum of each eluted compound and, therefore, to their identification when using the spectral libraries. There are several types of interfaces between the chromatograph and the mass spectrometer. The most developed ionization mode is electron impact (EI). From the total ion current (TIC), a chromatogram can be plotted which represents all of the compounds eluted. By choosing a particular ion ( selective ion monitoring , or SIM), a selective chromatogram can be produced. This method has become essential, notably in the context of environmental studies. Nevertheless, it requires the use of high‐performance columns ( ID = 0.1–0.2 mm) with no bleeding.
2.7.2 Selective Detectors
Nitrogen phosphorus detector (NPD)
This specific detector is sensitive to nitrogen (N) or phosphorus (P) compounds. It comprises a small ceramic cylinder doped with an alkaline salt (e.g. rubidium sulfate). A voltage is applied to maintain a small plasma (800°C) through the combustion of an air/hydrogen mixture ( Figure 2.12). Compared with FID, the flame is much smaller. Compounds containing nitrogen or phosphorus give, fairly specifically, decomposition fragments transformed into negative ions. These ions are then received by a collector electrode. The nitrogen present in air is inactive. Detector sensitivity is typically 0.1 pg/s for nitrogen‐ or phosphorus‐containing analytes, with a linear range of five orders of magnitude. However, it varies a lot with settings.
Electron capture detector (ECD)
This detector is considered to be selective because it is much more sensitive to halocarbon groups. A flow of nitrogen gas that has been ionized by electrons generated from a low‐energy β −radioactive source (a few mCi of 63Ni) passes between two electrodes maintained at a potential differential of around 100 V ( Figure 2.14). At rest, a base current I 0is generated, mainly due to free and very mobile electrons. If molecules ( M ) containing a halogen (F, Cl, Br) cross the zone between the two electrodes, they capture thermally excited electrons to form heavy negative ions, which by consequence are much less mobile.
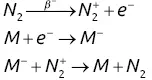
The measured intensity decreases exponentially by following a law of the type I = I 0exp[− kc ]. The linear range is about four orders of magnitude with nitrogen as the make‐up gas. The presence of a radioactive source in this detector means that it is subject to special regulations (inspection, location and maintenance visits). This detector is often used for analyses of chlorinated pesticides and polychlorinated biphenyls.
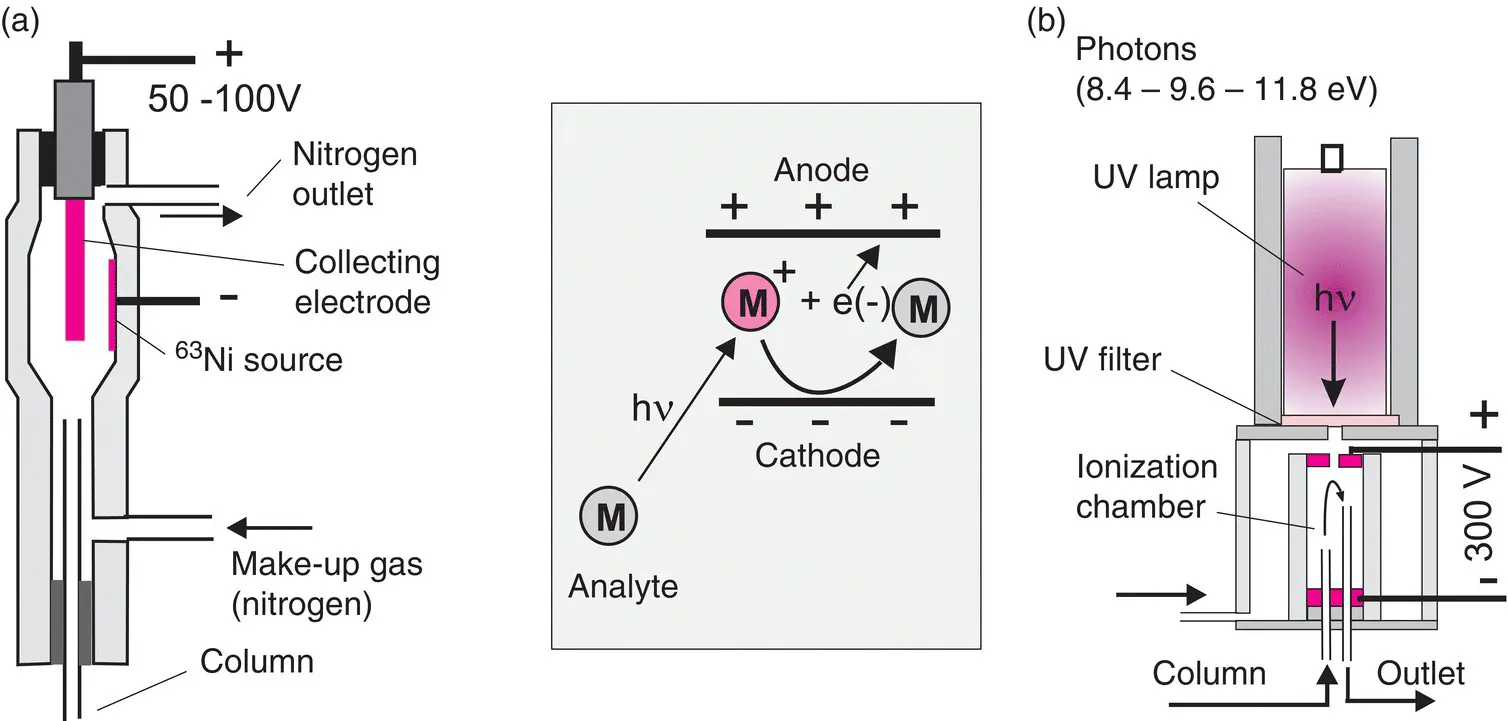
Figure 2.14 (a) Electron capture detector (ECD) and (b) photo‐ionization detector (PID). The PID contains a filter, used to select the photon energy in order for analytes to be the only ionized species apart from carrier gas molecules (LiF at 11.8 eV, or sapphire at 8.4 eV). The ionization mechanism is reversible.
Make‐up gas. To provide satisfactory response and increase their sensitivity, the above two detectors described above should be supplied with a gas flow of at least 20 ml/min, which is far greater than that in capillary columns. This flow rate is attained by mixing a make‐up gas with the gas coming out of the column outlet. This make‐up gas is either identical to the carrier gas or different from it (inexpensive nitrogen gas).
Photo‐ionization detector (PID)
This detector is fairly selective but it has only a narrow range of applications. It is suitable for hydrocarbons as well as for sulfur or phosphorus derivatives. The operating principle consists in provoking ionization of the analytes by irradiation with a UV lamp emitting high‐energy photons (8.4–11.8 eV). Photo‐ionization occurs when the energy of the photon is greater than the first ionization energy of the compound ( Figure 2.14). A photon of 9.6 eV can, for example, ionize benzene (PI 1= 9.2 eV) but not isopropanol (PI 1= 10.2 eV), which will therefore not be very visible on the chromatogram. Electrons released by an electrode connected to the terminal of an electrometer are collected for concentration measurements.
This detector can function at more than 400°C and is not destructive, as the ionization is reversible and affects only a small fraction of the molecules of each compound.
2.7.3 Detectors Providing Structural Data
None of the detectors previously described yield any information as to the nature of the eluted analytes. At most, they are selective for a certain category of compounds. Identification involves the use of an internal calibration based on retention times or requires the knowledge of retention indexes (see Section 2.10). When the chromatogram has peaks that are close together, a confusion of identity could occur.
To counteract this, several complementary detectors could be combined with each other ( Figure 2.15), or we could select a detector able to provide structural information based on spectroscopic data or about the elemental composition of the analytes. The retention time and specific characteristics for each compound can then be determined. The mass detector is the best example, yet other combinations of techniques have been tested. Such is the case for atomic emission detection with a plasma torch: compounds at the column outlet flow into a plasma (see Chapter 14) where the temperature is sufficient to increase atomic or ion populations in the excited state. When returning from the excited state, these atoms or ions emit specific radiations, which help to identify them. Tests have been conducted to couple GC with an infrared spectrometer (GC‐IR). This pairing leads in principle to mid‐infrared spectra of the eluted analytes. An entire set of technological adaptations have made the existence of coupled methods possible. Therefore, potential applications are more numerous than for each of these when taken alone.
Читать дальше




![Евгений Матерёв - Музеи… или вдохновляющая музыка The Chemical Brothers [litres самиздат]](/books/437288/evgenij-materev-muzei-ili-vdohnovlyayuchaya-muzyka-th-thumb.webp)







