Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia
Denis Y Logunov*, Inna V Dolzhikova*, Dmitry V Shcheblyakov, Amir I Tukhvatulin, Olga V Zubkova, Alina S Dzharullaeva, Anna V Kovyrshina, Nadezhda L Lubenets, Daria M Grousova, Alina S Erokhova, Andrei G Botikov, Fatima M Izhaeva, Olga Popova, Tatiana A Ozharovskaya,
Ilias B Esmagambetov, Irina A Favorskaya, Denis I Zrelkin, Daria V Voronina, Dmitry N Shcherbinin, Alexander S Semikhin, Yana V Simakova, Elizaveta A Tokarskaya, Daria A Egorova, Maksim M Shmarov, Natalia A Nikitenko, Vladimir A Gushchin, Elena A Smolyarchuk,
Sergey K Zyryanov, Sergei V Borisevich, Boris S Naroditsky, Alexander L Gintsburg, and the Gam-COVID-Vac Vaccine Trial Groupt
BackgroundA heterologous recombinant adenovirus (rAd)-based vaccine, Gam-COVID-Vac (Sputnik V), showed a good safety profile and induced strong humoral and cellular immune responses in participants in phase 1/2 clinical trials. Here, we report preliminary results on the efficacy and safety of Gam-COVID-Vac from the interim analysis of this phase 3 trial.
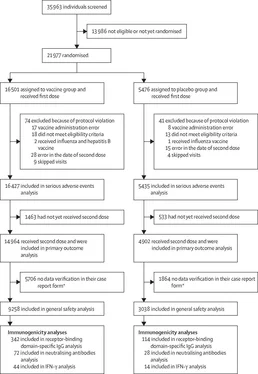
MethodsWe did a randomised, double-blind, placebo-controlled, phase 3 trial at 25 hospitals and polyclinics in Moscow, Russia. We included participants aged at least 18 years, with negative SARS-CoV-2 PCR and IgG and IgM tests, no infectious diseases in the 14 days before enrolment, and no other vaccinations in the 30 days before enrolment. Participants were randomly assigned (3:1) to receive vaccine or placebo, with stratification by age group. Investigators, participants, and all study staff were masked to group assignment. The vaccine was administered (0,5 mL/dose) intramuscularly in a prime-boost regimen: a 21-day interval between the first dose (rAd26) and the second dose (rAd5), both vectors carrying the gene for the full-length SARS-CoV-2 glycoprotein S. The primary outcome was the proportion of participants with PCR-confirmed COVID-19 from day 21 after receiving the first dose. All analyses excluded participants with protocol violations: the primary outcome was assessed in participants who had received two doses of vaccine or placebo, serious adverse events were assessed in all participants who had received at least one dose at the time of database lock, and rare adverse events were assessed in all participants who had received two doses and for whom all available data were verified in the case report form at the time of database lock. The trial is registered at ClinicalTrials.gov (NCT04530396).
FindingsBetween Sept 7 and Nov 24, 2020, 21 977 adults were randomly assigned to the vaccine group (n=16 501) or the placebo group (n=5476). 19 866 received two doses of vaccine or placebo and were included in the primary outcome analysis. From 21 days after the first dose of vaccine (the day of dose 2), 16 (0,1%) of 14 964 participants in the vaccine group and 62 (1,3%) of 4902 in the placebo group were confirmed to have COVID-19; vaccine efficacy was 91,6% (95% CI 85,6-95,2). Most reported adverse events were grade 1 (7485 [94,0%] of 7966 total events). 45 (0,3%) of 16 427 participants in the vaccine group and 23 (0,4%) of 5435 participants in the placebo group had serious adverse events; none were considered associated with vaccination, with confirmation from the independent data monitoring committee. Four deaths were reported during the study (three [<0,1%] of 16 427 participants in the vaccine group and one [<0,1%] of 5435 participants in the placebo group), none of which were considered related to the vaccine.
InterpretationThis interim analysis of the phase 3 trial of Gam-COVID-Vac showed 91,6% efficacy against COVID-19 and was well tolerated in a large cohort.
FundingMoscow City Health Department, Russian Direct Investment Fund, Sberbank, and RUSAL.
Copyright© 2021 Elsevier Ltd. All rights reserved.
The COVID-19 pandemic has led to more than 98 million confirmed cases and more than 2 million deaths (at the time of publication). There are a few provisionally licensed vaccines against COVID-19, and global efforts are focusing on developing safe and efficacious vaccines for COVID-19 prevention. According to the WHO draft landscape of COVID-19 candidate vaccines, 164 candidates
CrossMark
are in clinical assessment (including 13 at phase 3) and 173 are in preclinical analyses. The phase 3 vaccine candidates include a variety of vaccine platforms: vector vaccines (Gamaleya National Research Centre for Epidemiology and Microbiology [NRCEM; this study], University of Oxford/AstraZeneca, 2CanSino Biological Inc/Beijing Institute of Biotechnology, and Janssen Pharmaceutical Companies), mRNA-based vaccines
Published Online February 2, 2021 https://doi.org/10.10l6/S0140-6736(21)00234-8 See Online/Comment https://doi.org/10.1016/S0140-6736(21)00191-4 *Contributed equally tTrial group members are listed in the appendix Federal State Budget Institution “National Research Centre for Epidemiology and Microbiology named after Honorary Academician N F Gamaleya" of the Ministry of Health of the
Russian Federation, Moscow, Russia (D Y Logunov DSc,
I V Dolzhikova PhD,
D V Shcheblyakov PhD,
A I Tukhvatulin PhD,
0 V Zubkova PhD,
A S Dzharullaeva MSc,
A V Kovyrshina MSc,
N L Lubenets MSc,
D M Grousova MSc,
A S Erokhova MSc,
A G Botikov MSc,
F M Izhaeva MSc, O Popova MSc, T A Ozharovskaya MSc,
I B Esmagambetov PhD,
I A Favorskaya PhD,
D I Zrelkin MSc,
D V Voronina MSc,
D N Shcherbinin PhD,
A S Semikhin PhD,
Y V Simakova MSc,
E A Tokarskaya PhD,
D A Egorova PhD,
M M Shmarov DSc,
N A Nikitenko PhD,
V A Gushchin PhD,
Prof B S Naroditsky DSc,
Prof A L Gintsburg DSc); Federal State Autonomous Educational Institution of Higher Education I M Sechenov First Moscow State Medical University of the Ministry of Health of the Russian Federation (Sechenov University), Moscow, Russia (E A Smolyarchuk PhD,
Prof A L Gintsburg); Peoples' Friendship University of Russia (RUDN University), Moscow, Russia (S K Zyryanov DSc); 48 Central Research Institute of the Ministry of Defence of the Russian Federation, Moscow, Russia (S V Borisevich DSc) Correspondence to: Dr Denis Logunov, Federal State Budget Institution “National Research Centre for Epidemiology and Microbiology named after Honorary Academician N F Gamaleya" of the Ministry of Health of the Russian Federation, Moscow 123098, Russia ldenisy@gmail.com
See Online for appendix For the WHO COVID-19 dashboard see https://covid19.
who.int
Research in context Evidence before this study
We searched PubMed for research articles published up to Jan 25, 2021, with no language restrictions, using the terms "SARS-CoV-2" or "COVID-19", “vaccine", "clinical trial", and "efficacy". We found three peer-reviewed publications available on the efficacy of SARS-CoV-2 vaccines: AZD1222 (AstraZeneca/ University of Oxford), a ChAdOx1-based vaccine with reported efficacy of 70,4% and two mRNA-based vaccines: BNT162b2, (Pfizer/BioNTech) with reported efficacy of 95%, and mRNA-1273 (Moderna/NIAID), with reported efficacy of 94,1%. We have previously published safety and immunogenicity results of Gam-COVID-Vac in phase 1/2 clinical trials.
Added value of this study
We report on the interim clinical efficacy results of the rAd26 and rAd5 vector-based COVID-19 vaccine Gam-COVID-Vac in a randomised, double-blind placebo-controlled multicentre phase 3 trial in Moscow, Russia, including 21 862 participants.
Читать дальше