The primary outcome analysis included all participants who had received at least two doses at the time of database lock and followed protocol without violations. The analysis of serious adverse events included all participants who had received at least one dose at the time of database lock and followed protocol without violations. The safety analysis (including rare adverse events) included all participants who had received two doses and for whom all available data were verified in the case report form at the time of database lock. The statistical analysis was done using Stata, version 14, and GraphPad Prism, version 9.0. This trial is registered with ClinicalTrials.gov (NCT04530396).
Role of the funding source
The funder had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
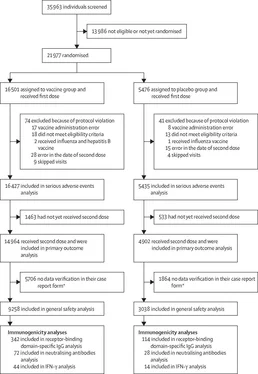
Between Sept 7 and Nov 24, 2020, 21977 adults were eligible and randomly assigned to receive placebo (n=5476) or vaccine (n=16 501; figure 1).
The first database lock was on Nov 18, 2020, when 20 cases of COVID-19 had been reported. The interim safety analysis (analysis of rare adverse events) was done with data up to the first database lock. Since there was an increase in COVID-19 incidence in Moscow during November, the second database lock was done on Nov 24, 2020, when 78 COVID-19 cases had been reported. Data for the interim efficacy analysis and serious adverse events analysis are presented up to the second database lock.
74 participants from the vaccine group and 41 from the placebo group were excluded from analyses (figure 1). This preliminary analysis included 16 427 participants in the vaccine group and 5435 in the placebo group, who received at least one dose and continued participation in the trial. 14 964 in the vaccine group and 4902 in the placebo group had received two doses at the time of database lock (Nov 24, 2020) and were included in the primary outcome analysis (table 1). Median time from participants receiving the first dose to the date of database lock was 48 days (IQR 39-58). Among the participants who received two doses, the mean age was 45,3 years (SD 12,0) in the vaccine group and 45,3 years (SD 11,9) in the placebo group; the distribution by sex (p=0,619), incidence of concomitant diseases (p=0,420), and infection risk (p=0,851) were similar between the two groups (table 1).
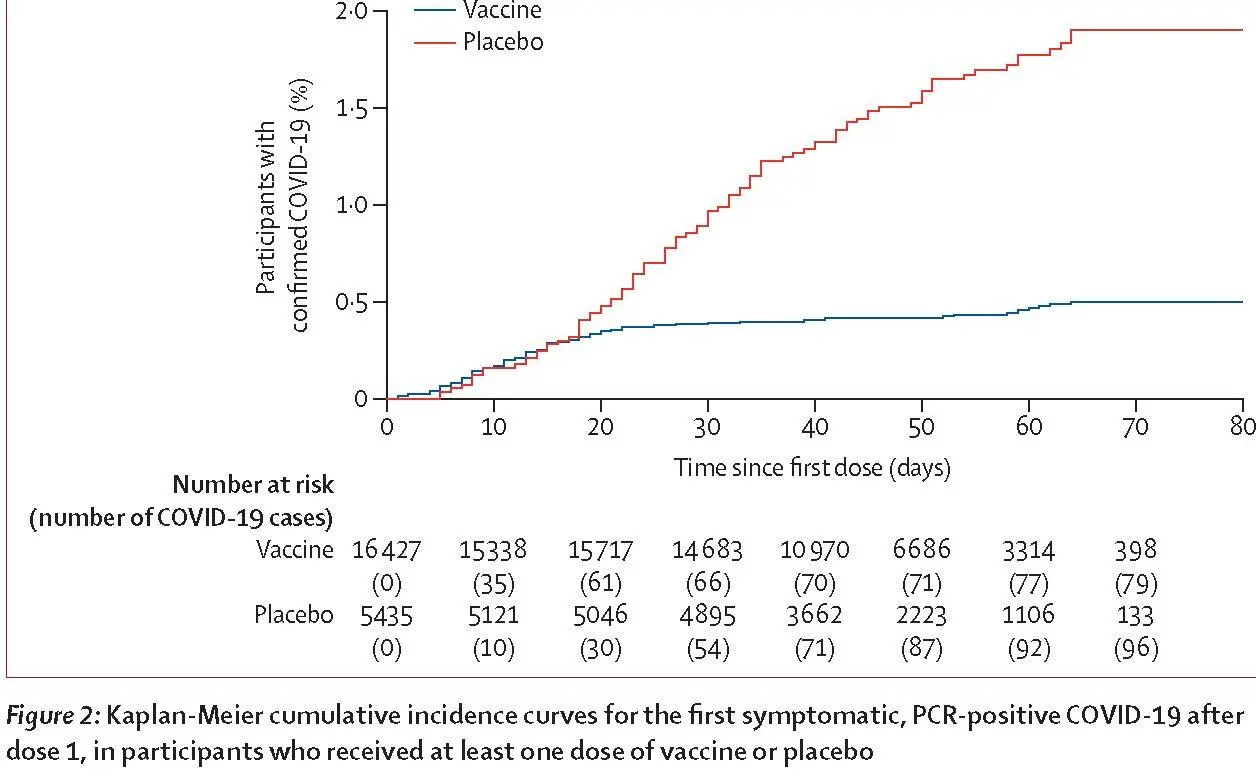
From 21 days after the first dose of vaccine (the day of dose 2), 16 COVID-19 cases were confirmed in the vaccine group (of 14964 participants; 0,1%) and 62 cases were confirmed in the placebo group (of 4902 participants; 1,3%); vaccine efficacy was 91,6% (95% CI
85,6-95,2; table 2). The observed vaccine efficacy was greater than 87% in all age and sex subgroups. Notably, vaccine efficacy was 91,8% (67,1-98,3) in participants older than 60 years. There were no cases (vaccine group) and 20 cases (placebo group) of moderate or severe COVID-19 confirmed at least 21 days after dose 1; thus, vaccine efficacy against moderate or severe COVID-19 was 100% (94,4-100,0). From 15 to 21 days after the first dose, efficacy was 73,6% (p=0,048), then from day 21, efficacy was 100% (p<0,0001; appendix p 11).
97 confirmed cases of COVID-19 (63 in the vaccine group and 34 in the placebo group) are not reflected in the analysis of the primary endpoint because they occurred fewer than 21 days after dose 1 (ie, before dose 2; table 2, figure 2). Estimated vaccine efficacy against confirmed COVID-19 occurring at any time after dose 1 was 73,1% (95% CI 63,7-80,1). Notably, in the vaccine group, most cases of COVID-19 occurred before dose 2. Rates of disease onset were similar for the vaccine and placebo groups until about 16-18 days after dose 1, after which, early onset of protection led to the number of cases in the vaccine group increasing much more slowly than in the placebo group (figure 2).
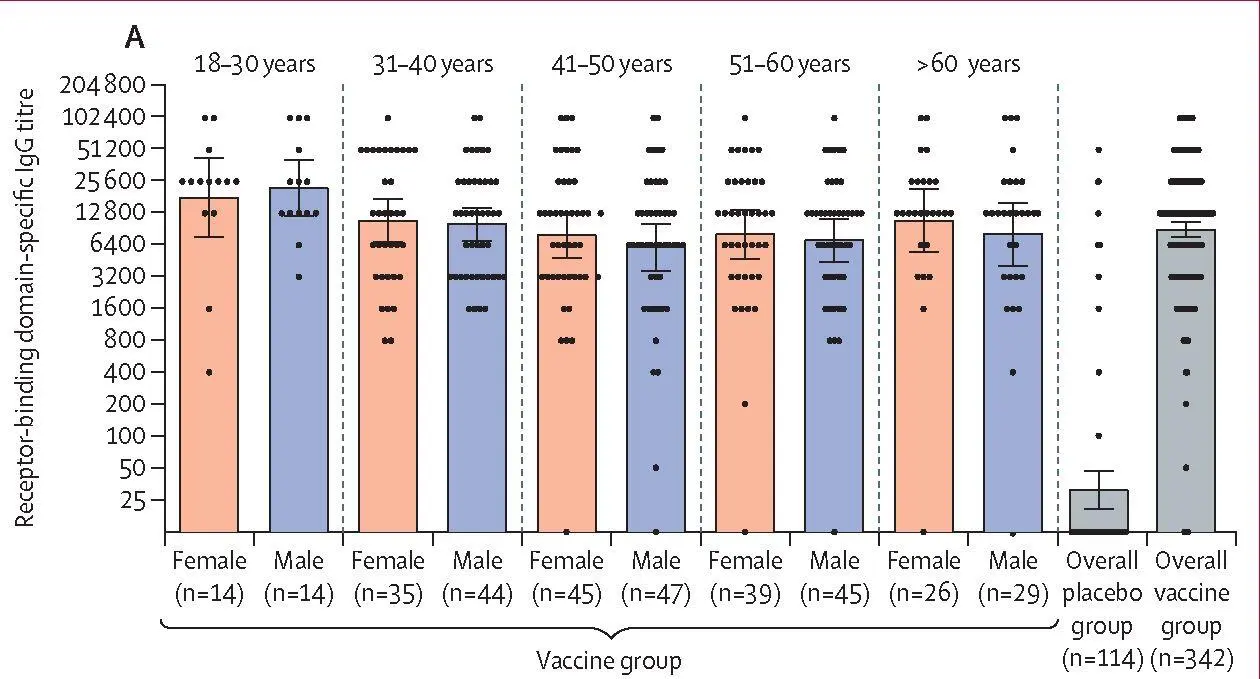
The interim immunogenicity analysis included samples transferred from the central laboratory that were collected before Nov 30, 2020, and showed that the vaccine induces an immune response in participants. Before first vaccination, no RBD-specific antibodies (of 456 participants tested) or virus-neutralising antibodies (of 100 participants tested) were detected in the blood serum of participants. In the analysis of humoral immune response, serum samples of 456 participants (342 from the vaccine group and 114 from the placebo group) were analysed for the presence of antibodies specific to the receptor-binding domain of SARS-CoV-2 glycoprotein S 42 days from the start of vaccination (figure 3A). In the vaccine group, RBD-specific IgG was detected in 336 (98%) of 342 samples, with a geometric mean titre (GMT) of 8996 (95% CI 7610-10 635), and a seroconversion rate of 98,25%. In the placebo group, RBD-specific IgG was detected in 17 (15%) of 114 samples, with a GMT of30,55 (20,18-46,26), and a seroconversion rate of 14,91% (p<0,0001 vs the vaccine group). When comparing the level of RBD-specific antibodies between age strata, we noted that the age 18-30 years group (combined male and female) had a significantly higher GMT than the other age groups (p=0,0065). There were no differences between the other age groups (p=0,343). Antibody levels did not differ significantly between men (n=179) and women (n=159; p=0,258). Descriptive statistics by age stratum and sex are in the appendix (p 3).
To assess the induction of a humoral immune response, serum samples from 100 participants were analysed for the presence of neutralising antibodies on day 42 after first vaccination (figure 3B); the GMT of neutralising antibodies was 44,5 (95% CI 31,8-62,2) and the seroconversion level was 95,83% in the vaccine
 |
| B |
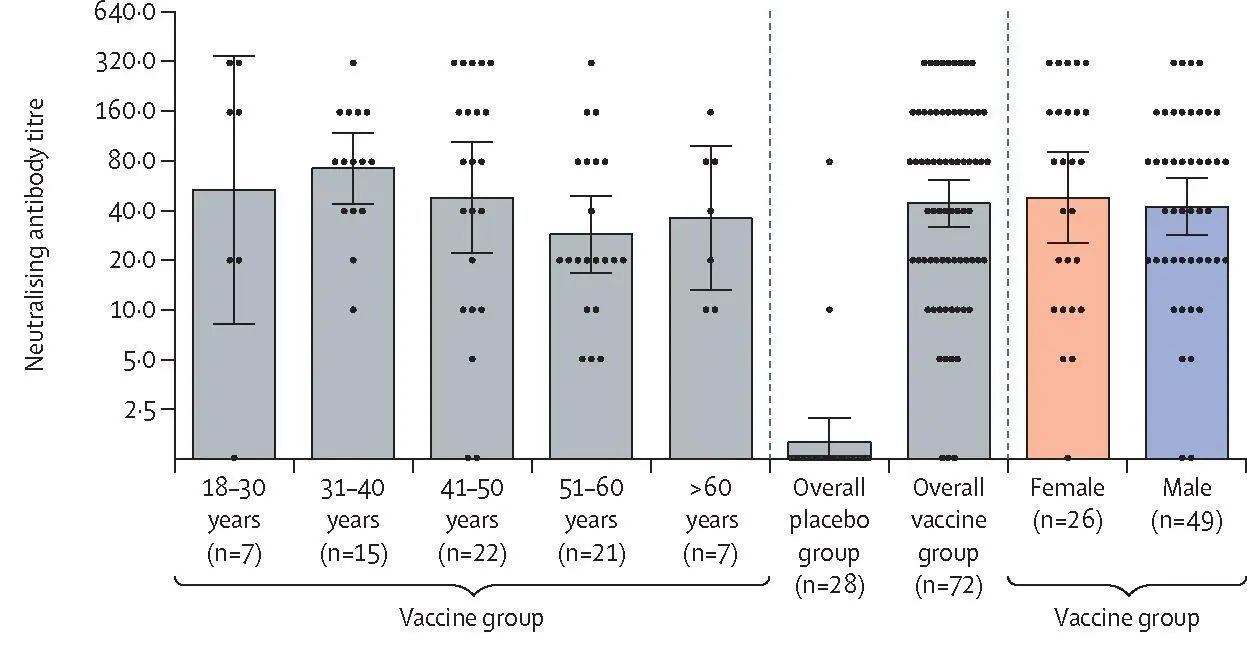 |
| Figure3: Humoral immune response(A) Receptor-binding domain-specific antibodies on day 42, as measured by ELISA, in participants administered with vaccine, by age group and overall, or placebo overall. (B) Neutralising antibodies on day 42, as measured by neutralisation assay with 100 TCID5 0, in participants administered with vaccine or placebo. Data are divided by age strata and by sex. Data of the overall vaccine group and placebo group are also presented. Dots show individual datapoints, bars show geometric mean titres, and whiskers show 95% CI. TCID 50=50% tissue culture infective dose. |
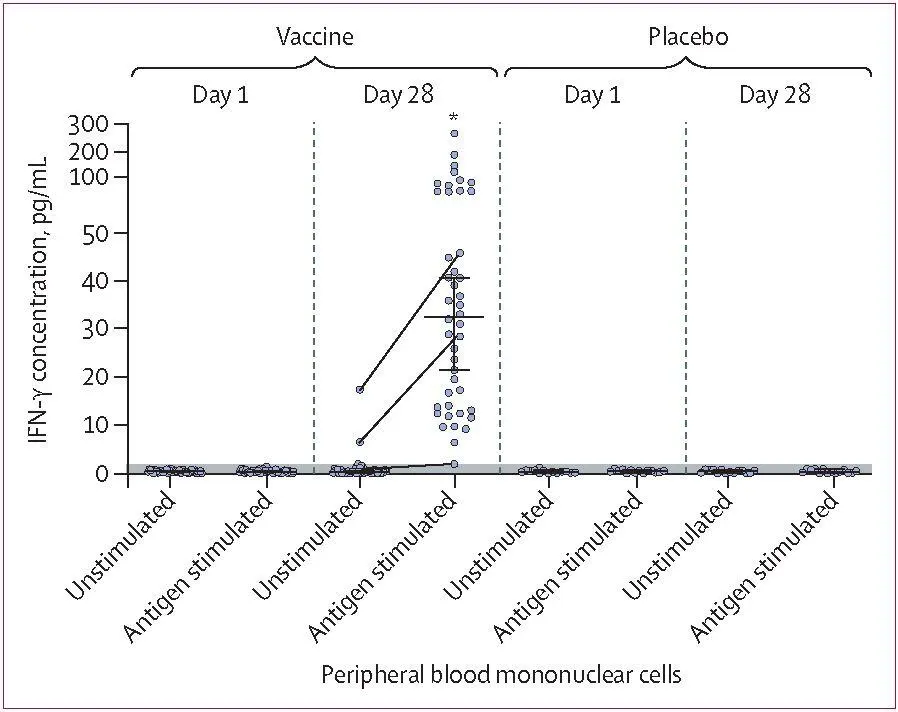 |
| Figure4: IFN-y response to SARS-CoV-2 glycoprotein S of peripheral blood mononuclear cells of participants who received two doses of vaccine (n=44) or placebo (n=14)Dots show individual datapoints of intact (unstimulated) cells and cells stimulated with SARS-CoV-2 glycoprotein S (antigen stimulated). Horizontal lines show median values, whiskers show 95% Cl. The threshold of detection (<2 pg/mL) is indicated with the grey shaded area. The grey lines connecting dots between unstimulated and antigen-stimulated cells show changes in IFN-y response in some representative individuals. *p<0'0001 for day 28 antigen-stimulated cells versus day 1 antigen-stimulated cells, in the vaccine group. |
group. GMT in the placebo group was 1,6 (112—2 19) and the seroconversion rate was 7,14%, which was significantly lower than that in the vaccine group (p<0,0001). Levels of neutralising antibodies were similar between age strata (p=0,222) and between men and women (p=0,639). Descriptive statistics by age stratum and sex are in the appendix (p 3).
Читать дальше