The vaccine comprises two vector components, rAd26-S and rAd5-S. A full dose of the vaccine was 10 11viral particles per dose for each recombinant adenovirus; 0,5 mL/dose for intramuscular injection. The placebo consists of the vaccine buffer composition, but without the recombinant adenoviruses, made up to equal the vaccine volume. Vaccine and placebo were developed, manufactured, and stored by Gamaleya NRCEM (Moscow, Russia) according to Good Manufacturing Practices. The vaccine and placebo were used in liquid form (frozen). The compositions of the vaccine and placebo are described in the appendix (p 1). The vaccine (first dose rAd26, second dose rAd5) or placebo were administered intramuscularly into the deltoid muscle with a 21-day interval between doses.
Subsequent observation visits were planned for day 28 (±2 days), day 42 (±2 days), and day 180 (±14 days). During the observation visits, vital signs were assessed in all trial participants and changes in the participants’ condition and wellbeing compared with the previous visit were recorded. A PCR test was done in combination with the clinical examination on the day of the second dose (day 21) for the diagnosis of symptomatic and asymptomatic COVID-19 cases. In the presence of clinical signs of respiratory infection and a positive PCR test, the participant would not be vaccinated with the second dose and was referred to medical staff for treatment of COVID-19. Participants without signs of respiratory infection were vaccinated before PCR results were received. In the case of a positive PCR test result, participants were classified as asymptomatic and were not counted as COVID-19 cases in the efficacy analysis, according to protocol. During the trial, apart from the screening visit and day of the second dose, no additional PCR tests were done, except when COVID-19 symptoms were reported by participants.
The sponsor arranged some additional observation visits remotely as telemedicine consultations. Unscheduled telemedicine consultations were encouraged for complaints or questions from participants about study procedures. All participants were given study team contacts at signing of informed consent and were instructed to contact the team on an as-needed basis, but
For the DM 365 MainEDC system
see https://datamanagement365.
com/services/
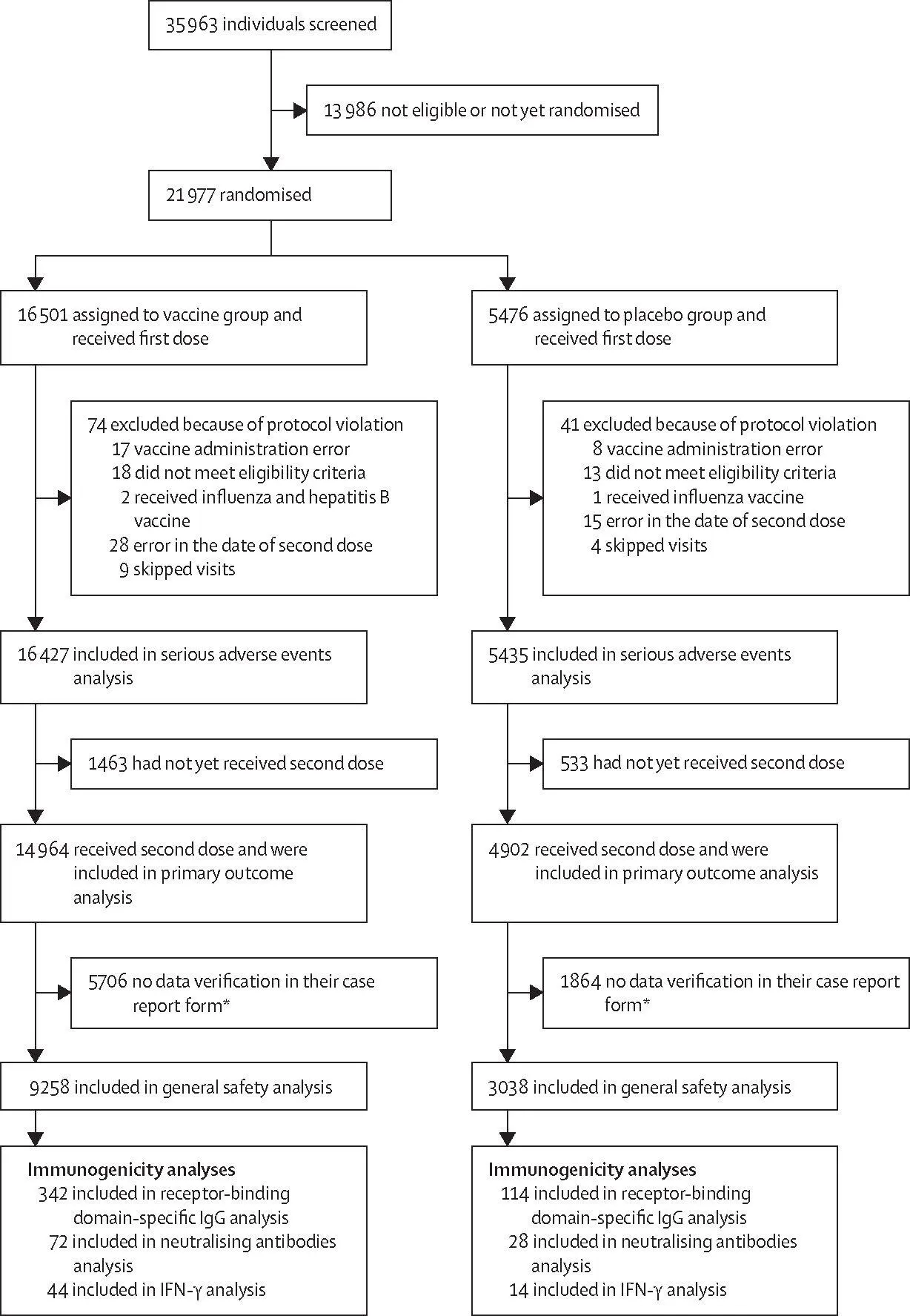 |
| Figure 1: Trial profile*At the time the database was locked, the data on adverse events in the case report form had not yet been verified in these participants; the data verification procedure can be done with a slight delay, thus, participants whose data were not verified were not included in this analysis. |
primarily to report any signs or symptoms that could be indicative of an adverse event. All participants were also offered electronic diaries to be installed on their smartphone devices to monitor their health status. Information from those participants who chose not to use e-diaries was collected by site staff via teleconsultation technology. Data collected from these telemedicine consultations were entered by the site investigators directly into the participant’s medical record.
A city-wide electronic health record (EHR) platform— the Unified Medical Information and Analytical System (UMIAS) is in place in Moscow. The UMIAS EHR is a controlled electronic medical record used by all Moscow health-care institutions for care provision to Moscow residents. EHRs of the trial participants were updated to indicate their participation in the trial and were used as a source for electronic data capture and source data verification by contract research organisation monitors. In addition to protocol-defined visits and teleconsultations, principal investigators and study teams were able to track patient status through this city-wide EHR platform, including possible hospital admission and use of ambulatory services. Electronic diaries from participants who agreed to use e-diaries were also integrated within the UMIAS EHR. For those participants who chose not to use e-diaries, data on participant status were collected by site staff via teleconsultations and entered into the EHR by site investigators. All adverse events were followed up by a clinical investigator until resolution and were reviewed by the data safety and monitoring board and verified by the trial monitor. When COVID-19 was suspected, participants were assessed according to COVID-19 diagnostic protocols, including PCR testing at a central laboratory in Moscow. Severity of disease was established upon confirmation of the COVID-19 diagnosis by site investigators. A description of the assessment criteria for severity of COVID-19 is in the appendix (p 2).
The study was organised and monitored by the Moscow branch of the Dutch contract research organisation Crocus Medical. Data management is done through the DM 365 MainEDC system(developed by Data Management 365), a powerful cloud-based platform integrated to comprise the functions of data collection, advanced randomisation techniques, full control over drug supply and dispensing, and patient e-diaries (electronic data capture, IWRS, drug supply, and electronic patient-reported outcomes). The system complies with all applicable international regulations, including Code of Federal Regulations Title 21 Part 11, Good Clinical Practice, Good Automated Manufacturing Practice 5, Health Insurance Portability and Accountability, and General Data Protection Regulation. The system allows collection and validation of clinical data in high-load clinical trials and supports central monitoring and risk-based monitoring processes, automated coding with the Medical Dictionary for Regulatory Activities (MedDRA), WHODrug, and Logical Observation Identifiers Names and Codes, and instant mapping of the exported data to the standard Clinical Data Interchange Standards Consortium Study Data Tabulation Model.
Blood sampling was done on the day of vaccination immediately before study drug administration. Blood sampling for assessment of immunogenicity parameters was only done in some study centres, selected on the basis of the logistics chain for the delivery of biomaterial to the central laboratory where primary blood processing was done (sera collection, aliquoting, and freezing).
Blood samples for antigen-specific IgG analysis are planned to be taken from up to 9520 trial participants before completion of the trial.
Immunogenicity was analysed as described previously. 12In brief, antigen-specific humoral immune response was analysed on the day of first vaccination and day 42. The titre of glycoprotein-specific antibodies in serum was ascertained by ELISA. To test anti-SARS-CoV-2 IgG, we used an ELISA that was developed at Gamaleya NRCEM and registered for clinical use in Russia (P3H 2020/10393 2020-05-18). The ELISA measures IgGs specific to the receptor-binding domain (RBD) of SARS-CoV-2 glycoprotein S. The titre of neutralising antibodies was measured on the day of first vaccination and day 42 by microneutralisation assay using SARS-CoV-2 (hCoV-19/Russia/Moscow_PMVL-1/2020) in a 96-well plate and a 50% tissue culture infective dose (TCID 50) of 100. The seroconversion rate was calculated as a four-fold increase in titre at 42 days compared with the day before first vaccination. Cell-mediated immune response was measured on the day of first vaccination and day 28 by quantification of IFN-y secretion upon antigen restimulation in peripheral blood mononuclear cell culture.
Outcomes
The primary outcome was the proportion of participants with COVID-19 confirmed by PCR from day 21 after receiving the first dose. The secondary outcomes were severity of COVID-19; changes in antibody levels against SARS-CoV-2 glycoprotein S; proportion of participants with antibodies against SARS-CoV-2 N-protein; changes in SARS-CoV-2 neutralising antibody titres (increase of titres); changes in antigen-specific cellular immunity level (increase of cell-mediated immune response to antigen); and incidence and severity of adverse events. Serious adverse events were diagnosed on the basis of the event requiring hospital admission. Here, we report preliminary results on the primary outcome measure, incidence and severity of adverse events, immunogenicity, and safety.
Читать дальше