Cellular immune response in participants was characterised by secretion of IFN-y of peripheral blood mononuclear cells upon SARS-CoV-2 glycoprotein S restimulation in culture. To assess cellular immune response, serum samples from 58 participants (44 from the vaccine group and 14 from the placebo group) were analysed. By day 28 after first vaccination, all participants in the vaccine group had significantly higher levels of IFN-y secretion upon antigen restimulation (median 32,77 pg/mL [IQR 13,94—50,76]) compared with the day of administration of the first dose (figure 4). Descriptive statistics for IFN-y immune responses are in the appendix (p 4).
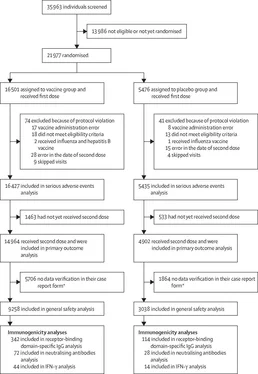
The general safety and rare adverse event analyses included 12 296 participants who received both doses up to the database lock on Nov 18, 2020. The most common adverse events were flu-like illness, injection site reactions, headache, and asthenia. Most of the reported adverse events (7485 [94,0%] of 7966) were grade 1; 451 were grade 2 (5,66%) and 30 were grade 3 (0,38%). 122 rare adverse events were reported in the study (91 in the vaccine group and 31 in the placebo group; appendix pp 8-9).
The analysis of serious adverse events included 21 862 participants who received at least one dose (of whom 19 866 received two doses) up to database lock on Nov 24, 2020. 70 episodes of serious adverse events, considered not related to COVID-19, were recorded in 68 participants: in 45 (0,3%) of 16 427 participants from the vaccine group and 23 (0,4%) of 5435 participants from the placebo group (appendix pp 5-7). None of the serious adverse events were considered associated with vaccination, as confirmed by the independent data monitoring committee (IDMC).
Because there were few serious adverse events, it was possible to process and verify the serious adverse events data up to the second database lock; however, full adverse events data, which has not yet been processed, will be provided in a later publication to avoid discrepancies with the final report after full data processing is complete.
During the study, four deaths were recorded: three (<0,1%) of 16 427 participants in the vaccine group and one (<0,1%) of 5435 participants in the placebo group. No vaccine-related deaths were reported. In the vaccine group, one death was associated with fracture of the thoracic vertebra and the other two were associated with COVID-19 (one patient with a severe cardiovascular background who developed symptoms on day 4 after first dose and one patient with a background of endocrinological comorbidities who developed symptoms on day 5 after first dose; appendix p 12). Based on the incubation period of the disease, both participants were deemed to be already infected before being included in the study, despite a negative PCR test. In the placebo group, the death was associated with haemorrhagic stroke.
The study included 2144 participants older than 60 years (1611 in the vaccine group and 533 in the placebo group). The mean age in this subgroup was 65,7 years (SD 4,5) in the vaccine group and 65,3 years (4,3) in the placebo group (appendix p 10). The maximum ages of the participants were 87 years in the vaccine group and 84 years in the placebo group. Proportions of participants by sex (p=0,378), incidence of concomitant diseases (p=0,774), and risk of infection (p=0,090) were similar between the vaccine and placebo groups. The vaccine was well tolerated in these participants. 1369 participants older than 60 years (who received two doses and for whom data in the case report form was verified at the time of database lock [Nov 18, 2020]) were included in the safety analysis. The most common adverse events were flu-like illness in 156 (15,2%) and local reaction in 56 (5,4%) of 1029 participants in the vaccine group and 30 (8,8%) and four (1,2%) of 340 participants in the placebo group (appendix p 11). There were three episodes of adverse events of grade 3 or worse, considered not associated with vaccination: an exacerbation of urolithiasis and acute sinusitis in the vaccine group and a flu-like illness in the placebo group. All these adverse events were resolved. In the participants older than 60 years, there were three serious adverse events reported in the vaccine group: renal colic and deep vein thrombosis (both associated with pre-existing comorbidities) and extremity abscess (due to physical injury and subsequent infection of the wound surface of the soft tissues of the finger). No association was found between serious adverse events and vaccine administration.
Our interim results of the phase 3 Gam-COVID-Vac trial show that the vaccine is 91,6% (95% CI 85,6-95,2) efficacious against COVID-19 (from day 21 after first dose, the day of receiving second dose). Our results also showed that the vaccine was 100% (95% CI 94,4-100) efficacious against severe COVID-19, although this was a secondary outcome so the results are preliminary. The vaccine was well tolerated, with 45 (0,3%) of 16 427 participants in the vaccine group reporting serious adverse events, all of which were considered not related to the vaccine. According to the study design, the starting point for counting COVID-19 cases for estimation of vaccine efficacy was 21 days after dose 1 (day of dose 2 administration). Although the study was not designed to assess the efficacy of a singledose regimen, our early starting point allows us to observe a possible partial protective effect of a single dose. The cumulative COVID-19 incidence curves of COVID-19 cases among the placebo and vaccine groups begin to diverge 16-18 days after the first immunisation, showing early onset of a partially protective effect after a single-dose immunisation; however, the study design does not allow us to draw conclusions from these observations.
The vaccine induced robust humoral (n=342) and cellular (n=44) immune responses in all age strata. Notably, there were a few non-responders in the vaccine group (six of 342), possibly due to immunosenescence in older people, individual characteristics of the formation of an immune response, or concomitant immunological disorders.
17 (15%) of 114 participants in the placebo group had RBD-specific antibodies on day 42, probably associated with asymptomatic COVID-19; however, none of these participants were SARS-CoV-2 PCR positive, nor did they report the onset of respiratory symptoms in the electronic diary or when interviewed as part of the telemedicine follow-up.
Given the importance of protecting populations at risk because of older age, we assessed the ability of the vaccine to induce an immune response and protect against COVID-19 in individuals older than 60 years. Our results show that the two-component vaccine Gam-COVID-Vac was able to induce a virus-neutralising humoral response in participants older than 60 years. Furthermore, vaccine efficacy in this group of participants did not differ significantly from the efficacy of the age 18-60 years group.
The limitations of the interim analysis of efficacy include the small sample sizes within age strata. Further data collection will allow for clarification of efficacy data within age groups. Furthermore, COVID-19 cases were detected through self report of symptoms by participants, followed by a PCR test, so only symptomatic cases of COVID-19 are included in the efficacy analyses.
Initially, we developed a vaccine in two forms: liquid (which is stored at -18°C) and freeze dried (which is stored at 2-8°C). In this study, we studied the liquid form of the vaccine that requires storage at -18°C. Storage at 2-8°C, a favourable temperature profile for global distribution, has been approved by the Ministry of Health of the Russian Federation.
We previously reported on the local and systemic post-vaccine adverse reactions of the Gam-COVID-Vac vaccine in a small sample of participants. 12In this interim analysis, we report serious adverse events in more than 21 000 participants (of whom more than 16 000 received the vaccine).
Читать дальше