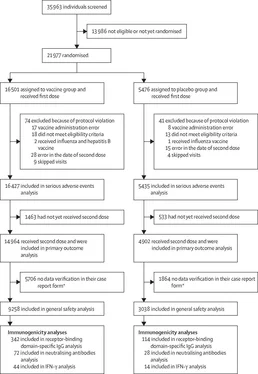
We describe the first immunogenicity results of the trial, including receptor-binding domain-specific IgG titres, virus neutralising antibody titres, and IFN-y response. The heterologous prime-boost regimen of vaccination provides robust humoral and cellular immune responses, with 91,6% (95% CI 85,6-95,2) efficacy against COVID-19. The vaccine is stored and distributed at -18°C, but storage at 2-8°C, a favourable temperature profile for global distribution, has also been approved by the Ministry of Health of the Russian Federation.
Implications of all the available evidence
A system-wide approach to stopping the COVID-19 pandemic requires the introduction of different vaccines based on different mechanisms of action to cover diverse global health demands with cost-effective and region-tailored methods.
Our vaccine, along with other SARS-CoV-2 vaccines, helps to diversify the world SARS-CoV-2 vaccine pipeline.
For the Moscow Government online platform see https:// www.mos.ru/
(Moderna/National Institute of Allergy and Infectious Diseases 3and BioNTech/Fosun Pharma/Pfizer 4),
inactivated vaccines (SinoVac, Wuhan Institute of Biological Products/Sinopharm, Beijing Institute of Biological Products/Sinopharm, and Bharat Biotech), and adjuvanted recombinant protein nanoparticles (Novavax).
The safety of adenoviral vector vaccines has been extensively studied, and adenoviral vector-based therapeutic drugs are used in clinical practice. 5-7Adenoviral vector-delivered antigens are known to induce both cellular and humoral immunity after a single immunisation, allowing their use as an emergency prophylaxis tool in a pandemic. Furthermore, the use of two immunisations gives a durable and long-lasting immune response. 8,9These characteristics make recombinant replication-deficient adenovirus (rAd)-based vaccines suitable candidates for the WHO target product profiles for long-term protection of people at high risk of COVID-19 in outbreak settings because they stimulate rapid onset of protective immunity. Although adenoviral vectors might induce immune responses against vector components and attenuate antigen-induced responses, prime-boost heterologous vaccination with two different vectors allows minimisation of this effect. 9-11Thus, the most effective approach for generating a powerful and long-lasting immune response that does not depend on the presence of a pre-existing immune response to the vector is the heterologous prime-boost vaccination approach. We used this approach when developing a vaccine for the prevention of COVID-19.
Gam-COVID-Vac is a combined vector vaccine, based on rAd type 26 (rAd26) and rAd type 5 (rAd5)— both of which carry the gene for SARS-CoV-2 full-length glycoprotein S (rAd26-S and rAd5-S). rAd26-S and rAd5-S are administered intramuscularly separately with a 21-day interval. The phase 1/2 clinical trials of the vaccine were completed in August, 2020. 12The results showed that the vaccine was well tolerated and highly immunogenic in healthy participants. As a result, the vaccine candidate was provisionally approved in Russia according to national legislation. Such registration allows the vaccine to be used in high-risk groups, with enhanced pharmacovigilance, while a post-marketing efficacy study is conducted. Here, we present preliminary efficacy and safety results of a phase 3 multicentre study using Gam-COVID-Vac in adults, with subanalysis of adults older than 60 years.
Study design and participants
This is a randomised, double-blind, placebo-controlled, multicentre, phase 3 trial to assess efficacy, immuno-genicity, and safety of the Gam-COVID-Vac combined vector vaccine against SARS-CoV-2-induced COVID-19 in adults, done at 25 hospitals and polyclinics in Moscow, Russia (appendix pp 14—15). Only sites accredited by the Ministry of Health of the Russian Federation for the conduct of clinical research were approved for participation. The trial protocol was reviewed and approved by appropriate competent authorities, including the Department of State Regulation for Circulation of Medicines of the Ministry of Health of the Russian Federation (approval number 450 from Aug 25, 2020), Moscow City Independent Ethics Committee, and independent local ethics committees of clinical sites.
The study used recruitment strategies that included use of the online platform of the Moscow Governmentand its call centres, community outreach, and recruitment efforts by approved clinical sites to achieve a high level of participation in the study. The study involved everyone who signed informed consent and passed screening.
Eligibility criteria were age 18 years or older; negative HIV, hepatitis B and C, and syphilis test results; negative anti-SARS-CoV-2 IgM and IgG antibody and SARS-CoV-2 PCR tests; no history of COVID-19; no contact with anyone with COVID-19 in the preceding 14 days; consent to use effective contraceptive methods; negative urine pregnancy test (for women of childbearing potential); negative drug and alcohol tests at screening visit; no history of vaccine-induced reactions; and no acute infectious or respiratory disease in the 14 days before enrolment.
Exclusion criteria were any vaccination in the 30 days before enrolment; steroids or immunoglobulins in the 30 days before enrolment; immunosuppression in the 3 months before enrolment; pregnancy or breastfeeding; acute coronary syndrome or stroke in the year before enrolment; tuberculosis or chronic systemic infections; allergy or hypersensitivity to the drug or components; neoplasms; blood donation in the 2 months before enrolment; splenectomy; neutropenia, agranulocytosis, significant blood loss, severe anaemia, or immunodeficiency in the 6 months before enrolment; active form of a disease caused by HIV, syphilis, or hepatitis B or C; anorexia or protein deficiency; large tattoos at the injection site; history of alcohol or drug addiction; participation in any other clinical trial; study centre staff or other employees directly involved in the trial or their families; or any other condition deemed a problem by the study physician. All participants provided signed informed consent to be included in the database for study participation.
Randomisation and masking
Enrolled participants were divided into five age strata (18-30 years, 31-40 years, 41-50 years, 51-60 years, and >60 years) and were assigned to two study groups using stratified (block size 4) interactive web response system (IWRS) randomisation in a ratio of 3:1 to the vaccine group or the placebo group. Study participants were assigned unique randomisation numbers that remained unchanged throughout the study. The statistician generated a sequence, according to which the drug was labelled. The drug and placebo were outwardly indistinguishable (packaging, label, and content). Investigators, participants, and all study staff were masked to group assignment.
Procedures
All participants who consented to participate attended a screening visit for physical examination, checks of vital signs (eg, blood pressure, heart rate, and temperature), and blood tests for infections (HIV, hepatitis B and C, and syphilis) and collection of baseline immunogenicity characteristics. Urine tests for drugs and alcohol were done in all volunteers and pregnancy tests were done in women. PCR SARS-CoV-2 swab tests were also done at screening by the central laboratory in Moscow to exclude participants with COVID-19. At screening, information on the presence of concomitant diseases and SARS-CoV-2 infection risk group was entered into the case report forms of the participants. High risk denotes those whose work involves interaction with patients with a confirmed diagnosis of COVID-19; medium risk is those who have professional contact with a large number of people, such as general practitioners, social workers, and shop assistants; and general risk denotes those with no additional risks associated with their professional activities. Intended duration of participation of individuals in the trial was 180 days after the first dose of the vaccine or placebo. One screening visit and five on-site visits to a clinical site over the course of the trial were planned.
Читать дальше