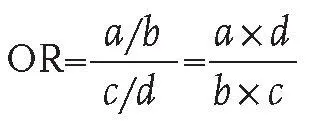Statistical analysis
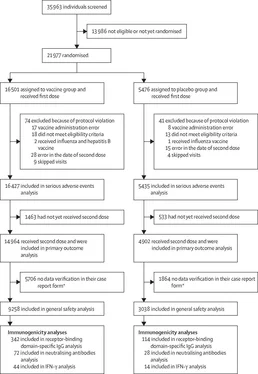
In this interim analysis, we present efficacy data at the point of confirmation of 78 COVID-19 cases in participants after receiving the second dose, as stipulated by the protocol.
In this study, the primary endpoint is the proportion of participants without COVID-19 confirmed by laboratory tests during the study. The frequency of COVID-19 in the general population, and thus the expected frequency in our placebo group, is 20 people per 1000 or 2,0%. The study aims to show that the proportion of participants with COVID-19 will be at least a third lower in the intervention group than the control group (odds ratio [OR] for the null hypothesis of 0,67)—ie, the upper limit of the 95% CI for the
OR should not exceed 0,67. The expected value of the effect is about 0,500 (OR for the alternative hypothesis of 0,500). With a planned study population of 40 000 participants and randomisation 3:1 vaccine to placebo, the study power will be 85%, with a unilateral statistical significance level of 0,025.
The study protocol did not originally prespecify a target number of events in this trial. However, because of the increase in the incidence of COVID-19 in Russia, changes were made to the clinical trial protocol on Nov 5, 2020, including an interim analysis to preliminarily calculate the vaccine efficacy and to establish ethical appropriateness of further inclusion of the placebo group in the trial in the context of a growing pandemic if the vaccine is effective. Three interim analyses were completed when 20, 39, and 78 documented cases of COVID-19 had occurred across both groups combined. Our original conservative estimate of efficacy was 50%. If the efficacy was at least 70%, then a statistically significant difference between the groups would be detected when at least 20 events were reached across the two groups. If efficacy was 65%, then the number of cases required would be 39. 60% efficacy would be statistically significant when 78 cases had been reported.
|
Vaccine (n=14 964) |
Placebo (n=4902) |
| Sex |
|
|
| Female |
5821 (38,9%) |
1887 (38,5%) |
| Male |
9143 (61,1%) |
3015 (61,5%) |
| Race |
| White |
14 741 (98,5%) |
4830 (98,5%) |
| Asian |
217 (1,5%) |
69 (1,4%) |
| Other* |
6 (<0,1%) |
3 (<0,1%) |
| Age group, years |
|
|
| 18-30 |
1596 (10,7%) |
521 (10,6%) |
| 31-40 |
3848 (25,7%) |
1259 (25,7%) |
| 41-50 |
4399 (29,4%) |
1443 (29,4%) |
| 51-60 |
3510 (23,5%) |
1146 (23,4%) |
| >60 |
1611 (10,8%) |
533 (10,9%) |
| Age, years |
45,3 (12,0) |
45,3 (11,9) |
| Bodyweight, kg |
81,3 (17,5) |
81,6 (17,7) |
| Height, cm |
173,1 (9,1) |
173,3 (9,0) |
| Body-mass index, kg/m 2 |
26,75 (4,56) |
26,75 (4,55) |
| Concomitant diseases (diabetes, hypertension, ischaemic heart disease, obesity)t |
3687/14 944 (24,7%) |
1235/4892 (25,2%) |
| Risk of infection in volunteers |
|
|
| High |
65/14 567 (0,4%) |
23/4778 (0,5%) |
| Medium |
3853/14 567 (26,5%) |
1280/4778 (26,8%) |
| General |
10649/14 567 (73,1%) |
3475/4778 (72,7%) |
| Data are n (%) and mean (SD). *Includes Black or African American, Native Hawaiian or other Pacific Islander, or undefined. Denominator shows number of participants for whom these data were available. High risk denotes those whose work involves interaction with patients with a confirmed diagnosis of COVID-19; medium risk is those who have professional contact with a large number of people, such as general practitioners, social workers, and shop assistants; and general risk denotes those with no additional risks associated with their professional activities. |
Table 1: Baseline characteristics of participants who received two doses of assigned treatment and were included in primary outcome analysis
The OR and 95% CI were calculated according to previously described methods. 13The primary endpoint was calculated using the following formula: vaccine efficacy (%)=(1 - OR) x 100, where the OR is as follows:
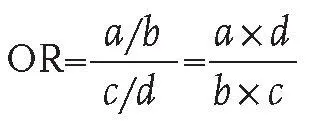
where a is the number of vaccinated participants with COVID-19, b is the number of vaccinated participants without COVID-19, c is the number of unvaccinated participants with COVID-19, and d is the number of unvaccinated participants without COVID-19.
ORs and 95% CIs were obtained by the Baptista-Pike method, p values were obtained by x 2test or Fisher’s exact test (if the expected frequency in any cell is <5). Cumulative incidence is presented using the Kaplan-Meier method.
In the safety analysis, adverse events were coded using MedDRA, version 23.0. Adverse events were presented by group, system organ and class, and preferred term. Normality of the data distribution was assessed with the d’Agostino-Pearson test in the analysis of quantitative data (immunogenicity analyses). In the analysis of immunogenicity (analysis of parametric data) in the case when two groups of data were compared, the Mann-Whitney U test was used (eg, the vaccine group vs placebo group or men vs women) for unpaired samples and the Wilcoxon signed rank test for paired samples (eg, cellular response data on days before and after vaccination). When comparing several groups of data
|
Totalcases |
Vaccine group |
Placebo group |
Vaccine efficacy (95% CI) |
p value |
| First COVID-19 occurrence from 21 days after dose 1 (day of dose 2)* |
| Overall |
78 |
16/14 964 (0,1%) |
62/4902 (1,3%) |
91,6% (85,6-95,2) |
<0,0001 |
| Age group (years) |
| 18-30 |
5 |
1/1596 (0,1%) |
4/521 (0,8%) |
91,9% (51,2-99,3) |
0,0146 |
| 31,40 |
17 |
4/3848 (0,1%) |
13/1259 (1,0%) |
90,0% (71,1-96,5) |
<0,0001 |
| 41-50 |
19 |
4/4399 (0,1%) |
15/1443 (1,0%) |
91,3% (73,7-96,9) |
<0,0001 |
| 51-60 |
27 |
5/3510 (0,1%) |
22/1146 (1,9%) |
92,7% (81,1-97,0) |
<0,0001 |
| >60 |
10 |
2/1611 (0,1%) |
8/533 (1,5%) |
91,8% (67,1-98,3) |
0,0004 |
| Sex |
| Female |
32 |
9/5821 (0,2%) |
23/1887 (1,2%) |
87,5% (73,4-94,2) |
<0,0001 |
| Male |
46 |
7/9143 (0,1%) |
39/3015 (1,3%) |
94,2% (87,2-97,4) |
<0,0001 |
| Moderate or severe cases |
20 |
0/14 964 |
20/4902 (0,4%) |
100% (94,4-100,0) |
<0,0001 |
| First COVID-19 occurrence after dose 1t |
| Any time after dose 1 |
175 |
79/16 427 (0,5%) |
96/5435 (1,8%) |
73,1% (63,7-80,1) |
<0,0001 |
| From 14 days after dose 1 |
109 |
30/14 999 (0,2%) |
79/4950 (1,6%) |
87,6% (81,1-91,8) |
<0,0001 |
| First COVID-19 occurrence after dose 2 (28 days after dose 1)* |
| All |
60 |
13/14 094 (0,1%) |
47/4601 (1,0%) |
91,1% (83,8-95,1) |
<0,0001 |
| Data are n/N (%), unless otherwise stated. *Includes those who received both doses. Includes participants who received at least one dose. |
| Table2: Interim results |
on vaccine efficacy |
|
|
|
(eg, age strata), the Kruskal-Wallis test was used. To compare the frequency indicators between groups, the x 2test and, if necessary, Fisher’s exact test were used (if the expected frequency in any of the cells was <5).
Читать дальше