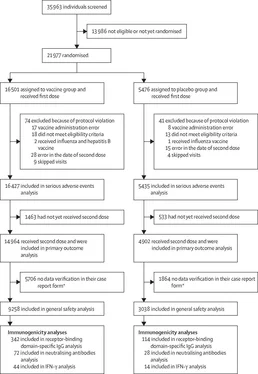
70 episodes of serious adverse events were recorded in 68 participants across the two groups; none of these events were considered related to the vaccine. During the study, four deaths were recorded: three in the vaccine group and one in the placebo group. None were considered related to the vaccine, with confirmation by the IDMC. No post-vaccination adverse events were reported in any of these participants after vaccination.
The two COVID-19-related deaths were due to preexisting cardiovascular and endocrinological conditions exacerbated by COVID-19. Taking into account the length of the incubation period described by WHO and the Centers for Disease Control and Prevention (CDC), 14,15these two participants were probably already infected with SARS-CoV-2 at the time of randomisation and vaccination. On the basis of WHO and CDC guidelines and review of the underlying clinical data, the IDMC confirmed that participants were infected and disease had progressed before any immunity from the vaccine developed. A detailed description of the condition of participants with COVID-19 is in the appendix (p 12). Among the seven participants assigned to the placebo group who were confirmed to have COVID-19 within the first 7 days after the first dose, there were no comorbid conditions, in contrast to the vaccine group, in which there were three participants with a comorbidity among 25 who were confirmed to have COVID-19 within the first 7 days.
In summary, both COVID-19-related deaths have several principal points to be considered. First, despite the negative PCR test at screening and absence of increased temperature at the time of first vaccine dose administration, the onset of the first COVID-19 symptoms (4-5 days after first dose, similar to the average COVID-19 incubation period) testifies that participants had been infected with SARS-CoV-2 before or near the vaccination day, which was additionally confirmed by the IDMC, on the basis of WHO and CDC guidelines and review of underlying clinical data. Second, both participants self-administered non-steroidal anti-inflammatory drugs without informing clinicians, which interfered with diagnosis and receipt of medical help upon hospital admission. Third, because of limited diagnostics at screening (limitations of medical examination and testing and patient unaware of comorbidities) each participant had comorbidities that were only known after hospital admission. Participants who have not developed protective immunity to SARS-CoV-2 (ie, were infected before vaccination or early after vaccination) showed the natural clinical course of COVID-19. We did another analysis of the severity of the course of COVID-19 in the two groups, which showed that in the first 2 weeks after the first dose, there was no significant difference in the severity of the course of COVID-19 between the vaccine and placebo groups. From 15 to 21 days after the first dose, efficacy was 73,6% (p=0,048), then from day 21, efficacy was 100% (p<0,0001; appendix p 11). Therefore, in this study, the efficacy analysis was done 21 days after the first dose, because by that time, the immune response is formed.
Currently, scrupulous monitoring continues, in particular for cases of COVID-19. All safety data will be provided to the regulator for analysis.
In this interim analysis, we have not been able to assess duration of protection; median follow-up time was 48 days after first dose. Although the study enrolled participants with comorbidities, not all risk groups are represented. There is a need to further investigate the vaccine in adolescents and children under Pediatric Investigational Plans, as well as pregnant and lactating women. Most participants in our trial were white, so we welcome further investigation in a more diverse cohort.
Interim results on efficacy have been announced for several vaccine candidates against SARS-CoV-2. The safety and efficacy study of the ChAdOx1 nCoV-19 vaccine (AZD1222) provides an analysis of data from four randomised controlled trials in Brazil, South Africa, and the UK. 11 636 participants were included in the primary efficacy analysis. Among participants who received two standard doses, the reported efficacy of the vaccine was 62,1%, and in participants who received a low dose followed by a standard dose, efficacy was 90,0%, resulting in overall efficacy of 70,4%. 2BNT162b2, an mRNA-based vaccine developed by Pfizer/BioNTech has reported 95% efficacy against COVID-19 in a multinational, placebo-controlled, observer-blinded, pivotal efficacy trial. 436 523 participants without baseline infection were included in the primary efficacy analysis. Eight cases of COVID-19 with onset at least 7 days after the second dose were observed in the study group and 162 cases were observed in the placebo group. There was one case of severe COVID-19 in a study group with onset at least 7 days after the second dose of BNT162b2. 4A phase 3, randomised, stratified, observer-blind, placebo-controlled study of an mRNA-1273 vaccine has enrolled 30 000 participants, 25% of whom are age 65 years or older. Interim results of the trial suggest efficacy of 94,1% based on 95 cases of asymptomatic COVID-19 among participants: 90 in the placebo group and five in the study group. 3The results of this Gam-COVID-Vac trial are not dissimilar to those reported for the other vaccines.
Vaccination strategies should account for a number of concerns regarding the priority of access to COVID-19 vaccines in various population groups, reliable risk assessment of adverse effects of vaccination in population groups with increased risk of severe COVID-19 (older adults and individuals with comorbidities), vaccine logistics (cold chain supply), sufficient coverage of immunisation, and duration of protective immune response. According to WHO target product profiles for COVID-19 vaccines, 16the characteristics required for emergency use during an outbreak include efficacy of at least 50%, suitability for use in older adults, maximum of two-dose regimen, and protection for at least 6 months. Further studies of candidate vaccines are needed to obtain information on duration of post-vaccine protective immune response. Yet, the results on efficacy and safety of COVID-19 vaccine candidates thus far are promising.
Our interim analysis of this phase 3 trial of Gam-COVID-Vac has shown promising results. In parallel with implementation of multiple clinical trials (in Russia, Belarus, United Arab Emirates, and India), the vaccine has already been released in Russia for use by the public, largely in at-risk populations, medical workers, and teachers, and as of Jan 23, 2021, more than 2 million doses of Gam-COVID-Vac have already been administered to the public (pharmacovigilance and monitoring of the incidence of rare adverse events is controlled by the Federal Service for Surveillance in Healthcare).
We are conducting research to investigate a singledose regimen of the vaccine (the clinical trial was approved by the Regulator and Ethics committee on Jan 8, 2021, number 1). Our interim analysis of the randomised, controlled, phase 3 trial of Gam-COVID-Vac in Russia has shown high efficacy, immunogenicity, and a good tolerability profile in participants aged 18 years or older.
Contributors
DYL is the principal investigator, performed research, and coordinated the study. IVD, DVS, AIT, and AVK drafted the manuscript. IVD, NLL, YVS, and EAT coordinated the study. IVD, OVZ, AIT, ASD, DMG, ASE, AVK, AGB, FMI, OP, TAO, IBE, IAF, DIZ, DVV, DNS, and ASS collected data. IVD, OVZ, AIT, YVS, EAT, NLL, DAE, NAN, MMS, and VAG contributed to the analysis and interpretation of the data. DYL, IVD, DVS, SVB, BSN, and ALG edited the manuscript. IVD and DVS did the statistical analysis. EAS and SKZ were involved in organisation, coordination, conduct, and technical support of the study. ALG was involved in organisation of research and the final decision to publish. All authors critically reviewed the manuscript and approved the final version. All authors had full access to all data in the studies and had final responsibility for the decision to submit for publication.
Читать дальше