Francis Rouessac - Chemical Analysis
Здесь есть возможность читать онлайн «Francis Rouessac - Chemical Analysis» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Chemical Analysis
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:3 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
Chemical Analysis: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Chemical Analysis»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
provides an up-to-date overview of the common methods used for qualitative, quantitative, and structural chemical analysis. Assuming no background knowledge in the subject, this student-friendly textbook covers the fundamental principles and practical aspects of more than 20 separation and spectroscopic methods, as well as other important techniques such as elemental analysis, electrochemistry and isotopic labelling methods.
Avoiding technical complexity and theoretical depth, clear and accessible chapters explain the basic concepts of each method and its corresponding instrumental techniques—supported by explanatory diagrams, illustrations, and photographs of commercial instruments. The new edition includes revised coverage of recent developments in supercritical fluid chromatography, capillary electrophoresis, miniaturized sensors, automatic analyzers, digitization and computing power, and more. Offering a well-balanced introduction to a wide range of analytical and instrumentation techniques, this textbook:
Provides a detailed overview of analysis methods used in the chemical and agri-food industries, medical analysis laboratories, and environmental sciences Covers various separation methods including chromatography, electrophoresis and electrochromatography Describes UV and infrared spectroscopy, fluorimetry and chemiluminescence, x-ray fluorescence, nuclear magnetic resonance and other common spectrometric methods such atomic or flame emission, atomic absorption and mass spectrometry Includes concise overview chapters on the general aspects of chromatography, sample preparation strategies, and basic statistical parameters Features examples, end-of-chapter problems with solutions, and a companion website featuring PowerPoint slides for instructors
, is the perfect textbook for undergraduates taking introductory courses in instrumental analytical chemistry, students in chemistry, pharmacy, biochemistry, and environmental science programs looking for information on the techniques and instruments available, and industry technicians working with problems of chemical analysis.
Review of Second Edition “An essential introduction to a wide range of analytical and instrumentation techniques that have been developed and improved in recent years.”
–

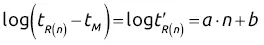
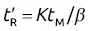 ; thus log
; thus log 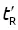 will increase with ln K for the homologous family: ln
will increase with ln K for the homologous family: ln 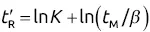 .
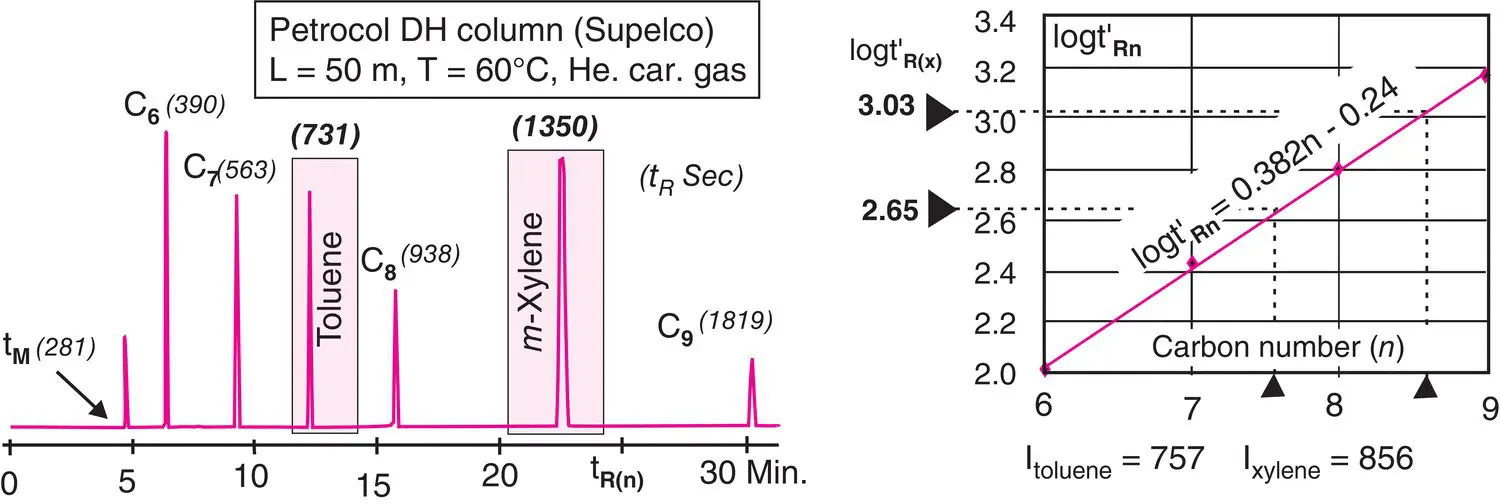
.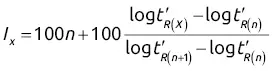
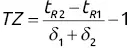
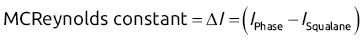


![Евгений Матерёв - Музеи… или вдохновляющая музыка The Chemical Brothers [litres самиздат]](/books/437288/evgenij-materev-muzei-ili-vdohnovlyayuchaya-muzyka-th-thumb.webp)







