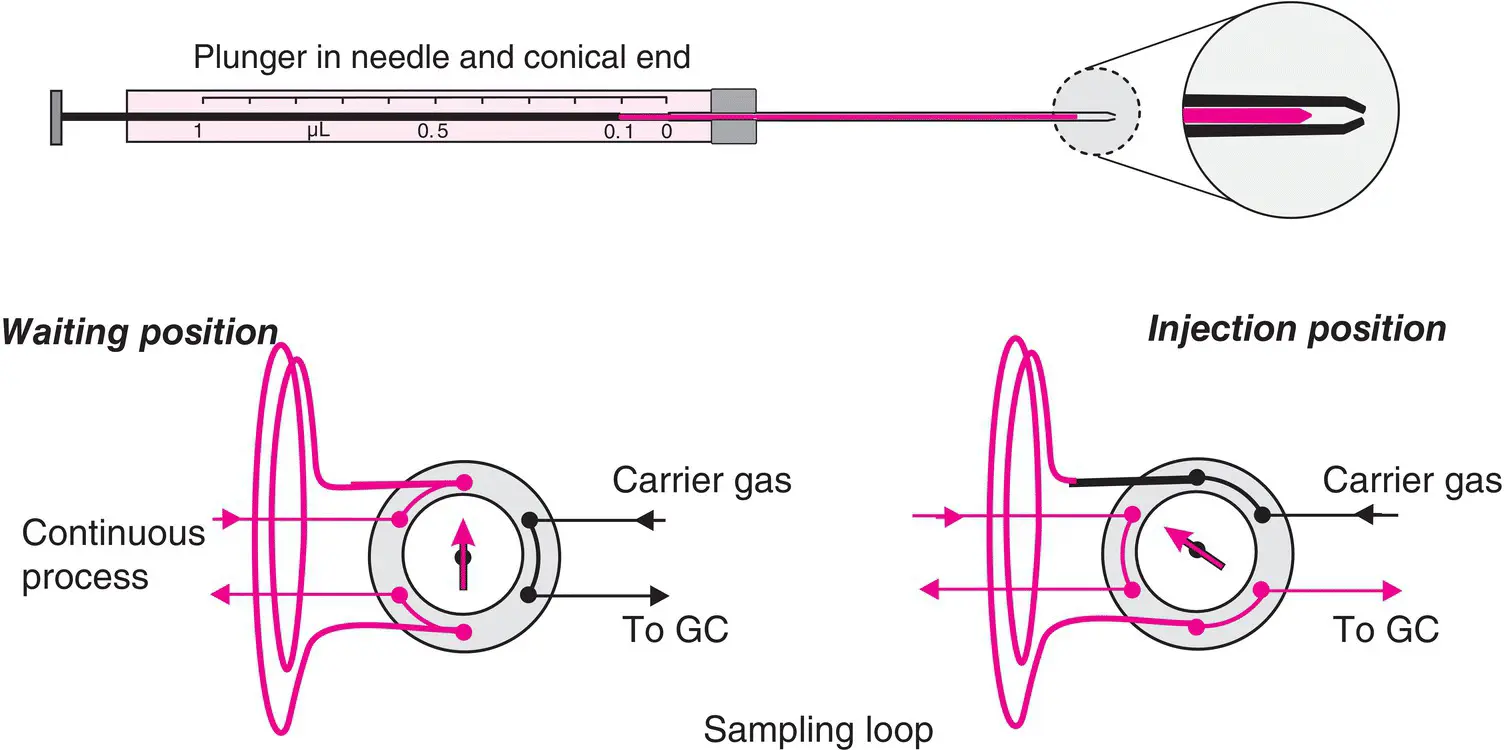
Figure 2.3 Microsyringe for GC and principle of an injection loop installed in a continuous process. The model chosen for this illustration has a cone shape, adapted to several septa or automatic injectors. In this model, the piston enters the needle to deliver the entire sample and prevent any dead volume. At the bottom, there is an example of an injection loop for gas or liquids (also see Figure 3.5).
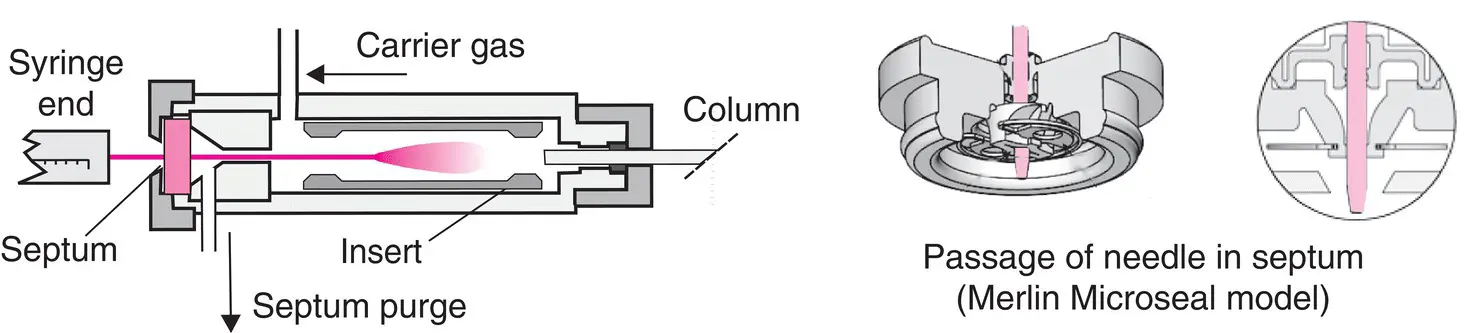
Figure 2.4 Direct vaporization injector used for packed columns. The typical septum is an elastomer disc but there are also more sophisticated versions, including the Merlin Microseal, which can be used thousands of times.
(Source: Courtesy of Agilent Technologies.)
Automatic injectors with a sample holder carousel are called autosamplers . With some of them, we can select the injection method (liquid, headspace, SPME (see Chapter 21)) and, if necessary, conduct a short preparation of each sample beforehand (dilution, addition of an internal standard, derivatization). These devices complete the chromatogram, improve results, and shorten analysis times. They have become essential for multi‐analyte methods in environmental analysis (search for PAHs, mycotoxins, pesticides).
When there is too little analyte to be detected (detectability limit) or to be quantified (quantification limit), we must make a preconcentration. To do so, we have such techniques as SPME (solid phase micro‐extraction) or SBSE (stir bar sorptive extraction). These techniques use the possibility of adsorbing an analyte on a solid phase, and inversely to desorb in the injector when subjected to heat (see Chapter 21).
For some applications, such as process control, there are injectors for gases or for liquids ( Figure 2.3), which include a loop, i.e. a small volume waiting and ready to be injected by rotation of a valve. We find this same principle in liquid chromatography (see Chapter 3, Section 3.3).
The injector, which is the sample’s entrance to the chromatograph, has different functions: to vaporize and entrain the sample mixed with the carrier gas at the head of the column. Depending on the way injection is conducted and on its rapidity, it can impact the quality of analysis.
Injectors differ depending on the nature of samples and types of analyses. Historically, the first injectors, called direct vaporization injectors, were intended for packed columns or macrobores, in which the carrier gas flow rate must be at least 6–7 ml/min. Any model of this type comprises a metal tube with a glass sleeve (called the insert ). It is swept by the carrier gas and heated to a sufficient temperature so that the less volatile solutes of the sample vaporize. The sample is introduced with a microsyringe, piercing the septum placed at the entrance of the injector ( Figure 2.4). The other end is connected directly to the column. Once all of the sample has been introduced with a syringe, it is immediately volatilized and enters the column, swept along by the carrier gas.
For capillary columns able to handle only a small capacity of sample, even the smallest volume that it is possible to inject with a microsyringe (0.1 μl) can saturate the column. Special injectors are used that can operate in two modes, with or without flow splitting (also called split or splitless ). This helps to adjust the sample fraction sent into the column as compared to the total quantity introduced in the chromatogram.
In split mode, a flow of carrier gas (e.g. 100 ml/min) arrives in the vaporization chamber where it mixes with the vapours of the injected sample ( Figure 2.5). A vent valve separates this flow into two fractions, of which the largest portion is vented from the injector, taking with it the majority of the sample introduced. The split ratio typically varies between 20:1 and 500:1. Only the smallest fraction, containing an amount of sample equal to the split ratio, will penetrate into the column.

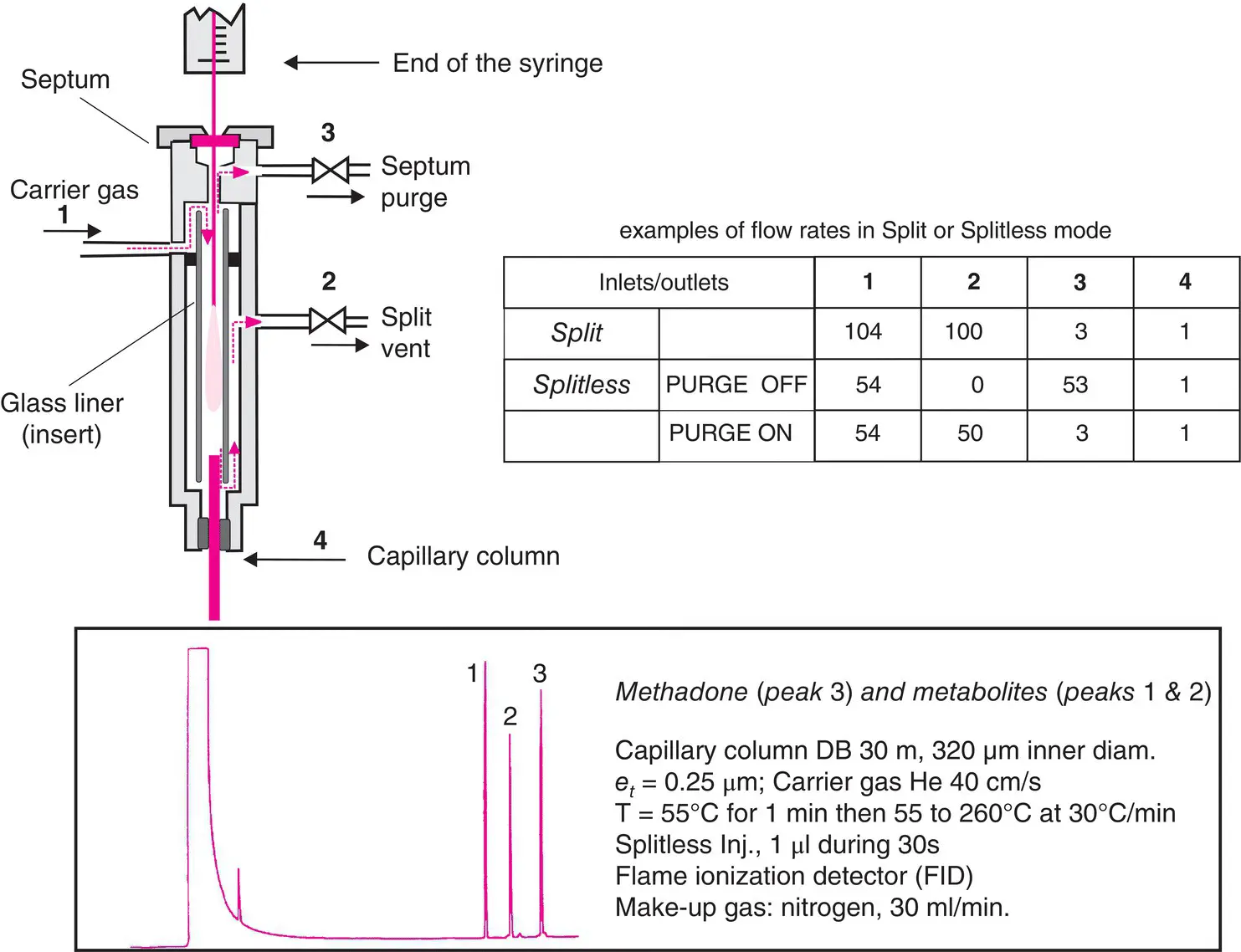
Figure 2.5 Injectors. Above left, injection chamber with splitter (vent 2 regulates the split ). Below, a typical chromatogram obtained in splitless mode. For solvent peaks that are superimposed upon those of the compounds, a selective detector which does not “see” the solvent is recommended.
Splitless mode is used only for very dilute samples. The contents of the microsyringe are injected very slowly from the microsyringe, during which bleeding valve 2 ( Figure 2.5) is maintained in a closed position for 0.5–1 minute in order for the vaporized mixture of compounds and the carrier solvent to be concentrated in the first few decimetres of the column, where the temperature is lower than the boiling point of the solvent. The opening of valve 2 provides an outlet for an excess of sample. The solvent precedes the compounds in the column and on the chromatogram. For either one of these injection techniques, chromatographs with software‐controlled parameters (flow rates and pressures) have a gas saver, which reduces the split ratio after the injection phase.
The split injector may lead to a poor evaluation of mixture composition, following a discrimination between compounds with varying volatilities: the composition of the fraction entering the column is not the same as the composition of the eliminated fraction. Therefore, it is a problem in quantitative analysis, where external standards are not advised.
Cold on‐column injection (COC)
The sample is injected directly into the capillary column ( cold on‐column or COC ). It is vaporized just after introduction. A special microsyringe, whose needle (steel or silica) is just 0.15 mm in diameter, is necessary for penetrating the column, which is cooled to 40°C before being allowed to return to its normal operating temperature. This process, useful for fragile compounds, is difficult to master in order to correctly eliminate the solvent(s). It is known not to discriminate between compounds of different volatilities ( Figure 2.6). The injector has a special duckbill septum. While it is useful for thermally labile compounds, there is a risk of column pollution if nonvolatile compounds are present.
Programmed temperature vaporization (PTV) injector
This PTV injector is considered to be the most universal. It can be used successively as a cold on ‐ column injector and then as a split/splitless injector. The temperature of the injection chamber can be programmed to create a gradient, e.g. from 20 up to 300°C, in a few tens of seconds ( Figure 2.6). So, the advantages of split/splitless injection are combined with those of cold injection onto the column. The advantages are:
Absence of discrimination due to the needle.
Use of classic syringes. Figure 2.6 PTV injector, with programmable temperature and cold on‐column injector. To make fast temperature gradients, the injection chamber is surrounded by a heating element or cooled by the circulation of a cold gas.
Читать дальше







![Евгений Матерёв - Музеи… или вдохновляющая музыка The Chemical Brothers [litres самиздат]](/books/437288/evgenij-materev-muzei-ili-vdohnovlyayuchaya-muzyka-th-thumb.webp)







