As with any scenario analysis there are some significant caveats. Given the complexity of the environment, it is difficult to draw lessons from one market and apply to another. Despite the aim for comparability of the markets and controlling for elements such as income levels, there are other key differences between the Argentinian economy and those of case studies chosen. These include resources for research (both human and capital), healthcare sector structure, strength of the economy, and social and cultural issues attached to innovation to name a few. Secondly, it is important to recognize that national activities can be subject to regional impacts, as investment has shifted towards Asia in recent years, independently of the policy framework in these markets (also largely driven by the Chinese economy).
However, we conclude that a strengthened environment for innovation would lead to a more dynamic biopharmaceutical industry and ensuing economic outputs. Indeed, it appears an ideal time to strengthen the environment building on the recent modest improvements in innovative friendly policy that have recently been introduced. This would lead to a compelling increase in levels of innovative activity across inputs (basic research and clinical trials) and outputs (patents) and lead to economic gains through higher sector employment, trade and taxes.
APPENDIX
Appendix 1 - Growth scenario analysis
Figure 6
Scenario analysis across innovative and economic activity in Argentina: Absolute gains and growth potential (final year)
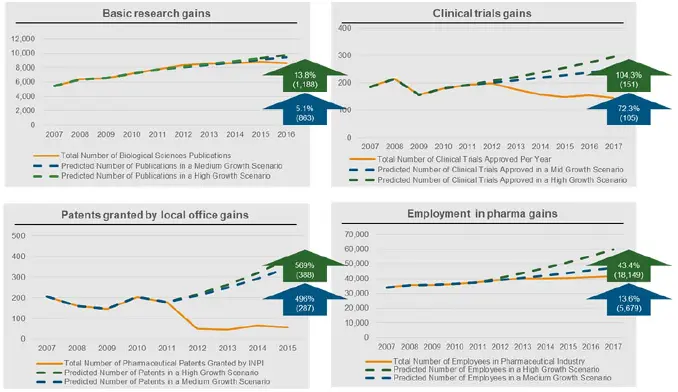
Source: CRA analysis
Appendix 2 - Statistical analysis
Table 4
Relationship between innovation regulation strength (proxied by patent index) to innovative and economic activity in four areas
| Variables |
Model 1(R&D spend) |
Model 2(Clinical Trials) |
Model 3(Patents) |
Model 4(Researchers) |
| Innovation regulation strength (ln_patent index) |
0.78**(0.34) |
2.58*(0.54) |
2.54*(0.87) |
1.77*(0.53) |
| Income level(ln_gdp_capita) |
0.02(0.16) |
0.63**(0.25) |
-0.13(0.38) |
0.43***(0.23) |
| F-Value |
5.49* |
42.70* |
6.83* |
19.35* |
| Number of observations |
49 |
49 |
47 |
42 |
Note: Value significant at: *1% level, **5% level, ***10% level.
Analysis: OLS multivariate regression analysis in a cross panel dataset (across countries).
Exploring the linkages between legal and marketing theories. Analysis of secondary patents in the pharmaceutical industry*
Galit Gonen Cohen
ABSTRACT
This research focused on an exploration of theoretical linkages between the fields of law and marketing. These linkages were explored by researching and analysing the role of secondary patents1 in the pharmaceutical industry, and more specifically, by investigating a new emerging practice which seems to exploit these linkages, according to which there is an attempt to extend the life-cycles of pharmaceutical products by filing secondary patents on the same active chemical ingredients as in the original drug products. This investigation was aimed at developing a new understanding of this emerging practice, which was then conceptualised to build up a theoretical model to explain the linkages between certain aspects of law and marketing.
The research consisted of three stages using qualitative and quantitative methods.
The findings indicate that patentability and validity of secondary patents are determined on a ‘case by case’ basis according to the law which sets they must comply with the universal requisites of novelty, industrial application and non-obviousness pre-determined criteria.
Regardless of the legal criteria, using secondary patents in marketing to extend product life-cycles is sometimes successful, depending on the specific circumstances of the case.
The conclusions apply the ‘Universalism-Particularism’ grand theory to the findings to suggest that while a pure universalistic approach mandates the field of law, a reconciliation approach dominates the interface between law and marketing and is thus the model offered for their linkage.
It is very interesting to note that the evidence reveals that although the practice of filing secondary patents in an attempt to extend the life-cycle of a product was started by innovative companies, the innovative companies gradually abandoned this route over the years. Generic companies picked it up and started to file this kind of patents.
RESUMEN
Esta investigación se centró en una exploración de los vínculos teóricos entre los campos del derecho y la comercialización. Estos vínculos se exploraron investigando y analizando el papel de las patentes secundarias2 en la industria farmacéutica y, más específicamente, investigando una nueva práctica emergente que parece explotar estos vínculos, según el cual existe un intento de extender los ciclos de vida de los productos farmacéuticos mediante la presentación de patentes secundarias sobre los mismos ingredientes químicos activos que en los medicamentos originales. Esta investigación tuvo como objetivo desarrollar una nueva comprensión de esta práctica emergente, que luego se conceptualizó para construir un modelo teórico para explicar los vínculos entre ciertos aspectos del derecho y el marketing.
La investigación consistió en tres etapas utilizando métodos cualitativos y cuantitativos. Los hallazgos indican que la patentabilidad y la validez de las patentes secundarias se determinan “caso por caso” según cumplan o no con los requisitos de la ley: novedad, altura inventiva (no ser obvio) y aplicación industrial.
Independientemente de los criterios legales de carácter universal, el uso de patentes secundarias en la comercializción para extender los ciclos de vida del producto puede a veces ser exitoso, dependiendo de las circunstancias particulares del caso.
Las conclusiones aplican la teoría del ‘Universalismo-Particularismo’ a los hallazgos para sugerir que, si bien el campo del derecho exige un enfoque universalista puro, un enfoque de reconciliación domina la interfaz entre la ley y el marketing y, por lo tanto, es el modelo ofrecido para su vinculación.
Es muy interesante observar que la evidencia revela que aunque las empresas innovadoras iniciaron la práctica de registrar patentes secundarias en un intento de extender el ciclo de vida de un producto, las compañías innovadoras gradualmente abandonaron esta ruta a lo largo de los años. Son las empresas que comercializan los llamados medicamentos genéricos las que comenzaron a presentar este tipo de patentes secundarias.
ARTICLE
a) Introduction
As seen in everyday life, innovative pharmaceutical companies attempt to promote their markets using legal strategies. In particular, companies use sophisticated patent strategies with the aim of extending their products’ market exclusivity and profitability in order to maximise their return on investment, and to justify research and development (‘R&D’) expenditures. They thus utilise patent strategies to pursue patent term extensions - either using the traditional provisions of extending the patent term to recover time lost in the regulatory approval process or employing more creative ways such as filing secondary patents. Furthermore, it appears that pharmaceutical companies apply for secondary patents in relatively large numbers and litigate them with tenacity.
Generally, the value of a patent is determined by the competitive advantage it confers and by the length of time this advantage exists. The value of secondary patents on the same active ingredient has not been sufficiently researched. Thus, the effectiveness of filing secondary patents in extending the product life-cycle and expanding the patented term has not yet been established, even though multiple entities such as pharmaceutical companies, courts and governmental offices (such as the US Patent Office) already devote significant resources to the issue of secondary pharmaceutical patents. A new understanding of this practice may thus have both theoretical and practical benefits. Furthermore, new theoretical insights on this practice may offer a basis for a theoretical explanation of the linkage between the fields of law and marketing, as this research combines concepts from these fields in an original manner.
Читать дальше













