Overall, Argentina lags behind other countries in the region and comparable OECD and Asian markets in many measures of innovative activities. It performs below peers in private investment in R&D, clinical research outputs and patents issued locally. However, this does not reflect its capacity to undertake innovative activity, as it leads in human capital, infrastructure and strength of the healthcare system. There is potential to unlock further value from existing resources in undertaking research activities and increasing the levels of investment, which remain suboptimal comparatively to other countries in LatAm.
Table 2
Comparison of Argentina to Latin America and the OECD
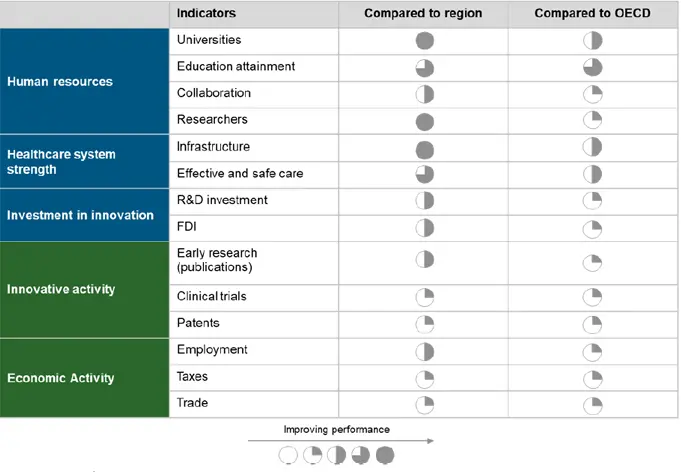
Source: CRA Analysis
3.3. The evidence from Taiwan and South Korea
The focus of this analysis is to investigate the potential impact of policy changes such as the introduction of innovation plans on the biopharma industry and the development of data protection regulation. These reflect the perceived areas of weaknesses in the Argentinian system. We focus on two case study countries that have undertaken significant efforts in changing the environment in these areas.
First, drawing on South Korea, the most significant changes for the purposes of this study (and hence relevance to Argentina) were the successive national S&T plans such as Bio-Vision 2016 and the 577 Initiative as set out below:71
The 577 plan, launched in 2008, outlined three overarching objectives: (1) Invest 5% of GDP on R&D (2) Focus on 7 key S&T areas and (3) Be of the 7 major S&T powers. The focus on 7 S&T areas is divided between 7 R&D areas (key industrial technologies, emerging industrial technologies, knowledge-based service technologies, state-led technologies, national issues-related technologies, global issues-related technologies and basic & convergent technologies) and 7 systems (world-class human resources, basic & fundamental research, SME (small and medium enterprise) innovation, S&T globalization, regional innovation, S&T infrastructure and S&T culture).
As a continuation of the progress made with Biotech 2000, in 2007, South Korea embarked on Bio-Vision 2016; the second phase of their biotechnology promotion plans. One of the major achievements of Biotech 2000 was that of increased South Korean competitiveness from a publication and patent standpoint. South Korea moved from 29th in 1994 in SCIE study rankings to 13th in 2005. With regards to patents, South Korea increased in the US Technical Strength rankings from 21st in 1994-1996 to 14th in 2003 to 2005 and since 1998 the number of Korean domestic patents filed far outstripped foreign patents.72
These innovation policies were introduced in parallel to changes in data protection. The Pharmaceutical Affairs Act (PAA), initially launched in 2007, includes a provision that new and certain prescription drugs may benefit from a de facto data protection period of four or six years as there is no centralised data exclusivity system. An additional data protection benefit in South Korea is that all biologic drugs benefit from de facto data exclusivity.73
Figure 3
Innovation policies and IP rules in South Korea
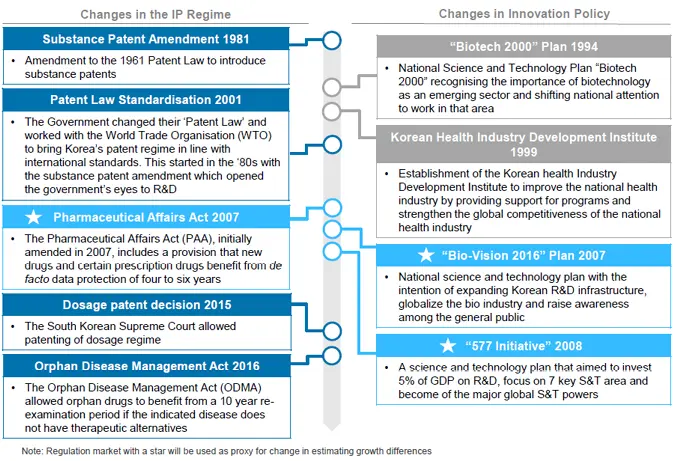
Source: CRA Analysis. Note: Star indicates key events considered in the analysis.
The second case study country, Taiwan, provides an example of a country where IP incentives were gradually introduced, and at the same time, supplemented with a strong biopharmaceutical industrial strategy to develop a strong and innovative sector. Pharmaceutical inventions have been patentable in Taiwan since the introduction of a law in 1968 that extended patent protection to include pharmaceutical and chemical products.74 Additional IP incentives for pharmaceutical products - Supplementary Protection Certificates (SPCs) were adopted in 1997.75 Following the joining of WTO in 2002, Taiwan implemented additional TRIPS regulations and introduced 5-year long RDP. The grant of RDP is contingent upon filing for marketing approval in Taiwan within three years of obtaining marketing authorisation in a different country.76 At that time, data exclusivity did not apply to new dosages, formulations, indications and combinations.77 In line with period of available data across the indicators and allowing for sufficient time for an impact to be observed, we have selected the first introduction of RDP in 2005 as a key event in the regulatory IP regime, whose impact we are investigating in the follow-on analysis. It should be noted that it was not until 2017 that Taiwan adopted RDP for new indications.
In terms of industrial innovation plans and incentives, we focus on amendments to the national plan on biotech industry released in 2003. This aimed to establish Taiwan as the centre for genomic research and the leading location for clinical trials, as well as the most vibrant biotech-focused venture capital industry in the Asia Pacific region.78 As illustrated in Figure 4, the Biotech and Pharmaceutical Technology Island plan consisted of three major projects: (1) the National Health Information Infrastructure Plan, (2) building Taiwan’s Biobank database and (3) establishing a clinical trial and research system.79 Around the same time, Taiwan implemented regulations to facilitate the knowledge transfer from academia into industry, whose impact on innovation should also be accounted for. For example, Taiwan’s Biotech and New Pharmaceutical Development Act from 2007 allowed publicly-funded researchers to help private companies with R&D.
Figure 4
Innovation policies and IP rules in Taiwan

Source: CRA Analysis. Note: Star indicates key events considered in the analysis.
To understand the impact of changes in the policy environment we have used these events in each country as an anchor to review what happened to measures of innovative activity before and after the introduction of the policy. In reality, changes in innovative activity occur slowly over time and it is challenging to attribute any changes to a single policy (but rather the joint effect of the programme of initiatives). Therefore, we have applied a wide window, looking for an impact during the five years after the introduction of the policy starting around the second year after the policy change. There are also other changes ongoing in terms of market potential, the policy environment in other parts of the world that would ideally be taken into account. However, it is noticeable that examining the many of the metrics we can observe a change in the level or a change in the growth rate following the introduction of these policy initiatives. In addition, we have looked at the local literature and the degree to which government, academia or grey literature has attributed the change in the metric to the change in policy environment. Whilst the literature examining their impact is scarce, two notable exceptions indicate that:
The progressive changes in protection provided in South Korea are seen as important component in incentivising innovative activity. Before this, most pharmaceutical companies did not conduct large amounts of innovative R&D but there has been an industry-wide shift occurred to focus more intensely on innovative R&D.80
It was reported that despite opposition from the local pharmaceutical associations, consensus was reached that RDP would stimulate innovative activities and amendments to the Pharmaceutical Affairs Law in Taiwan were introduced in February 2005.81
Читать дальше















