(Notice that the boundaries of the box were shifted from 0 to L to − L /2 to + L /2 for symmetry reasons that will be taken up again in Section 3.2.) The Schrödinger equation is written in two parts: Inside the box, where the potential energy is zero, the same equation holds that was used earlier:
(2.23) 
Outside the box, the Schrödinger equation is
(2.57) 
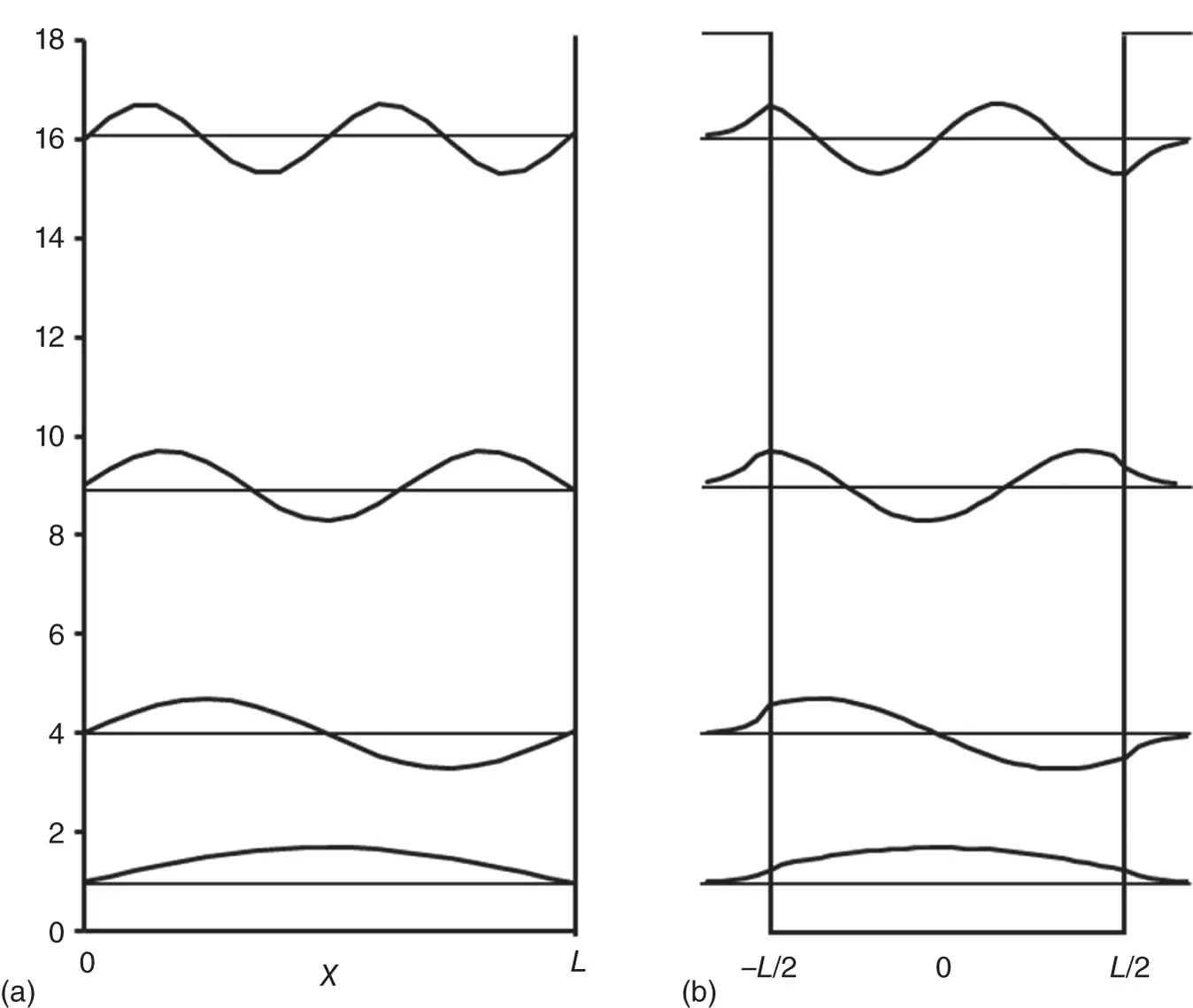
Figure 2.5 (a) Particle in a box with infinite potential energy barrier. (b) Particle in a box with infinite potential energy barrier.
The solutions of this equation will be of the form
(2.58) 
(2.59) 
where
(2.60) 
For 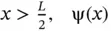 is an exponential decay function, and for
is an exponential decay function, and for 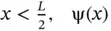 is an exponential growth function. This is shown in Figure 2.5bto the right and left of the potential energy box, respectively. This represents the probability of finding the electron outside the box, a process that is known as “tunneling.” Inside the box, the solutions of Eq. (2.23)resemble the bound wavefunctions of the particle in a box, except that the amplitude at the boundary is no longer zero, but must meet with the wavefunction outside the box. This is depicted in Figure 2.5. Bound states exist for energies E ( n ) < V 0only; for E ( n ) > V 0, the electron exists as a traveling wave as discussed before for unbound states.
is an exponential growth function. This is shown in Figure 2.5bto the right and left of the potential energy box, respectively. This represents the probability of finding the electron outside the box, a process that is known as “tunneling.” Inside the box, the solutions of Eq. (2.23)resemble the bound wavefunctions of the particle in a box, except that the amplitude at the boundary is no longer zero, but must meet with the wavefunction outside the box. This is depicted in Figure 2.5. Bound states exist for energies E ( n ) < V 0only; for E ( n ) > V 0, the electron exists as a traveling wave as discussed before for unbound states.
The concept of tunneling may seem esoteric at first, but it has interesting consequences. For example, a technique exists that is known as “tunneling electron microscopy (TEM)” where a very sharp metal tip is moved very close (within fractions of a nanometer) to the surface of the analyte, which is at a positive potential with respect to the metal tip. A tunneling current is observed between the tip and the analyte that is due to electrons tunneling from the tip to the analyte. As the substrate is moved laterally under the tip, the tip is lowered or raised to keep the tunneling current constant. In this way, an “image” of the morphology of the analyte can be obtained. Tunneling may also play a role in certain chemical reactions that depend on electron transfer from a donor to a receptor; some of these reactions are faster than expected from computations of the reaction rate from the activation energy. It is thought that in these reactions, the electron may tunnel from donor to receptor at a very fast rate. Finally, in the last example of “real‐world PIBs” in the section below, tunneling plays a major role.
2.5 Real‐World PiBs: Conjugated Polyenes, Quantum Dots, and Quantum Cascade Lasers
2.5.1 Transitions in a Conjugated Polyene
Although the PiB was introduced here as a model to demonstrate quantum mechanical principles in a mathematically manageable system, there are several physical examples that can be treated adequately using the PiB formalism. One of these is frequently incorporated as an experiment in physical chemistry laboratories [2] and involves a conjugated dye such as 1,6‐diphenyl‐1,3,5‐hexatriene, shown in Figure 2.6a. In this molecule, the Lewis structure suggests three double and four single bonds in the link between the two phenyl groups. From the viewpoint of the PiB formalism, one may consider the polyene framework the length of the box, indicated by the straight line connecting the two phenyl groups, and the six π‐electrons to be delocalized over the entire conjugated length and constituting six electrons in a box in three electron pairs. A schematic of the π‐bonding scheme is shown in Figure 2.6b. The three electron pairs would, in this model, occupy the n = 1, n = 2, and n = 3 levels, as indicated by the up/down arrows in each of these levels. The absorption spectrum in the visible range shows one absorption peak that is, in this approximation, assigned as a PiB transition of one electron from the highest occupied molecular orbital (HOMO) with n = 3 to the lowest unoccupied molecular orbital (LUMO) with n = 4, as indicated by the heavy up arrow. In Example 2.4, the wavelength of this absorption will be calculated. This example may be a bit premature in this chapter, because it introduced aspects of transitions between energy levels, principles of bonding orbitals, and so forth. These subjects will be taken up in later chapters in more detail, but this example shows quite nicely that the PiB formalism can be applied to real systems. In some experiments in physical chemistry laboratory, the dependence of the absorption wavelength on the “length of the box,” that is, the conjugated length, has been described.

Figure 2.6 (a) Structure of 1,6‐diphenyl‐1,3,5‐hexatriene to be used as an example for the PiB calculations. (b) Energy level diagram, based on the PiB formalism, showing the three lowest energy levels occupied by the π‐electrons.
Example 2.4Calculation of the energy difference between n = 3 and n = 4 energy levels for the 1,6‐diphenyl‐1,3,5‐hexatriene system, shown in Figure 2.6, assuming that the electrons obey the particle in a box formalism. What is the wavelength of a photon that causes this transition?
Answer:
1 Estimation of the conjugated length. Since the single and double bonds, with bond lengths of 154 pm and 130 pm, respectively, are approximately 120o from each other, one can approximate the length of the box as(E2.4.1)
2 Calculation of the energy difference between n = 3 and n = 4. Use me = 9.1×10−31 [kg] and h = 6.6 × 10−34 [Js] for the electron mass and Planck's constant. Since the length of the box was estimated to 2 significant figures, the entire computation is carried out with 2 significant figures:
Analysis of units:
(E2.4.2) 
Δ E = 3.7 × 10 −19[J]

Figure 2.7 Absorption spectra of nanoparticles as a function of particle size. As expected, the larger particles exhibit lower energy (longer wavelength) transitions.
Читать дальше







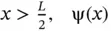 is an exponential decay function, and for
is an exponential decay function, and for 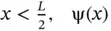 is an exponential growth function. This is shown in Figure 2.5bto the right and left of the potential energy box, respectively. This represents the probability of finding the electron outside the box, a process that is known as “tunneling.” Inside the box, the solutions of Eq. (2.23)resemble the bound wavefunctions of the particle in a box, except that the amplitude at the boundary is no longer zero, but must meet with the wavefunction outside the box. This is depicted in Figure 2.5. Bound states exist for energies E ( n ) < V 0only; for E ( n ) > V 0, the electron exists as a traveling wave as discussed before for unbound states.
is an exponential growth function. This is shown in Figure 2.5bto the right and left of the potential energy box, respectively. This represents the probability of finding the electron outside the box, a process that is known as “tunneling.” Inside the box, the solutions of Eq. (2.23)resemble the bound wavefunctions of the particle in a box, except that the amplitude at the boundary is no longer zero, but must meet with the wavefunction outside the box. This is depicted in Figure 2.5. Bound states exist for energies E ( n ) < V 0only; for E ( n ) > V 0, the electron exists as a traveling wave as discussed before for unbound states.













