1 Cover
2 Title Page Infrared Spectroscopy Set coordinated by Pierre Richard Dahoo and Azzedine Lakhlifi Volume 4
3 Copyright First published 2021 in Great Britain and the United States by ISTE Ltd and John Wiley & Sons, Inc. Apart from any fair dealing for the purposes of research or private study, or criticism or review, as permitted under the Copyright, Designs and Patents Act 1988, this publication may only be reproduced, stored or transmitted, in any form or by any means, with the prior permission in writing of the publishers, or in the case of reprographic reproduction in accordance with the terms and licenses issued by the CLA. Enquiries concerning reproduction outside these terms should be sent to the publishers at the undermentioned address: ISTE Ltd 27-37 St George’s Road London SW19 4EU UK www.iste.co.uk John Wiley & Sons, Inc. 111 River Street Hoboken, NJ 07030 USA www.wiley.com © ISTE Ltd 2021 The rights of Pierre Richard Dahoo and Azzedine Lakhlifi to be identified as the authors of this work have been asserted by them in accordance with the Copyright, Designs and Patents Act 1988. Library of Congress Control Number: 2021938325 British Library Cataloguing-in-Publication Data A CIP record for this book is available from the British Library ISBN 978-1-78630-652-4
4 Foreword
5 Preface
6 1 IR Spectra in Space Observation
1.1. Introduction
1.2. Fourier transform spectroscopy
1.3. Resonant cavity laser absorption spectroscopy
1.4. Spectroscopy for space observation
1.5. Conclusion
1.6. Appendices
7 2 Interactions Between a Molecule and Its Solid Environment
2.1. Introduction
2.2. Active molecule – solid environment system
2.3. Two-center expansion of the term
2.4. Conclusion
2.5. Appendices
8 3 Nanocage of Rare Gas Matrix
3.1. Introduction
3.2. Rare gases in solid state
3.3. Molecule inclusion and deformation of the doped crystal
3.4. Motions of NH 3trapped in an argon matrix
3.5. Infrared spectra
3.6. Appendices
9 4 Nanocages of Hydrate Clathrates
4.1. Introduction
4.2. The extended substitution model
4.3. Clathrate structures
4.4. Inclusion of a CH 4or NH 3molecule in a clathrate nanocage
4.5. System Hamiltonian and separation of movements
4.6. Translational motion
4.7. Vibrational motions
4.8. Orientational motion
4.9. Bar spectra
4.10. Appendices
10 5 Fullerene Nanocage
5.1. Introduction
5.2. Ammonia molecule trapped in a fullerene C 60nanocage
5.3. Potential energy surfaces – inertial model
5.4. Quantum treatment
5.5. Bar spectra
5.6. Appendices
11 6 Adsorption on a Graphite Substrate
6.1. Introduction
6.2. “NH 3molecule–substrate” system interaction energy
6.3. Equilibrium configuration and potential energy surfaces
6.4. Hamiltonian of the system
6.5. Infrared spectra of the NH 3molecule adsorbed on the graphite substrate
6.6. Conclusion
6.7. Appendices
12 References
13 Index
14 End User License Agreement
1 Chapter 1 Figure 1.1. Interferometer diagram. For a color version of this figure, see www....
Figure 1.2. Experimental setup for laser spectroscopy: induced fluorescence and ...
Figure 1.3. Experimental setup of the cryostat head
Figure 1.4. FTIR spectrometer and experimental setup. For a color version of thi...
Figure 1.5. O3 region ν3: (a) HF double site and (b) BF simple site (gas: 1042.0...
Figure 1.6. CO2 region ν2: (a) BF stable site and (b) HF unstable site (gas: 648...
Figure 1.7. N2O region ν2: double site (gas: 588.77 cm–1)
Figure 1.8. Nd-Yag laser: (1) cavity bottom mirror (Rmax = 1,064 nm), (2) shutte...
Figure 1.9. Dye laser, cavities, optical elements and mixer crystal LiNbO3
Figure 1.10. Global fluorescence: (a) stable site and (b) unstable site, Ar/13CO...
Figure 1.11. Double resonance signals: (a) carried by the line P26 (A: 4.2 µs) a...
Figure 1.12. Effect of gain saturation for an inhomogeneous widening: hole burni...
Figure 1.13. Diagram of a CRDS setup and temporal profile of the optical signal ...
Figure 1.14. Temporal profile of the transmission of a laser pulse and spectral ...
Figure 1.15. CW-CRDS by injection through resonance [ROM 97a]: P, piezoelectric;...
Figure 1.16. Pulse train emitted by a laser source in the temporal range Figure 1.17. Pulse train emitted by a laser source providing a frequency comb in...Figure 1.18. Elements of an ellipsometer: S, source; P, polarizer; λ/4, quarter ...Figure 1.19. Rough surface modeled by an effective medium. For a color version o...Figure 1.20. Maxwell–Garnett and Bruggeman mean-field models. For a color versio...Figure 1.21. Maxima and minima of a function. For a color version of this figure...Figure 1.22. Ellipsometric parameters Ψ (Psi) and Δ (Delta) on the spectral rang...Figure 1.23. Optical constants n and k in the 370–900 nm range. For a color vers...Figure 1.24. Optical constants n and k of tholins between 400 nm and 1,000 nm fo...Figure 1.25. Absorption spectra of tholins in mid-infrared for various initial c...Figure 1.26. Comparison of optical constants k of tholins for various initial co...Figure 1.27. Optical diagram of UV and IR channels of SPICAM light. (1) UV chann...Figure 1.28. Optical diagram of SOIR: (1) entrance optics, (2) diaphragm, (3) AO...Figure 1.29. Diagram of the LIDAR operating principle. For a color version of th...Figure 1.30. Diagram of LIDAR measurement parameters. For a color version of thi...Figure 1.31. Diagram of the Doppler wind LIDAR observation principle. For a colo...
2 Chapter 2Figure 2.1. Geometric characteristics of a symmetric top NH3 molecule in interac...
3 Chapter 3Figure 3.1. Curves of the normalized density of phonon states | (solid line curv...Figure 3.2. Inclusion diagram, in a simple substitution site, of the NH3 molecul...Figure 3.3. Level curves of the potential energy surfaces (cm-1): | (on the left...Figure 3.4. Orientational level scheme attached to species A (k = 0) and E (k = ...Figure 3.5. Inversion–orientation bar spectrum of NH3 in an argon matrix calcula...Figure 3.6. Vibration–inversion–orientation bar spectrum of NH3 in an argon matr...Figure 3.7. Spectral profile of the inversion–orientation of NH3 in an argon mat...Figure 3.8. Spectral profile of the vibration–inversion–orientation of NH3 in an...
4 Chapter 4Figure 4.1. LS state and HS state of Fe(II) and Fe(III) ions. For a color versio...Figure 4.2. Small and large cages forming the sI and sII structures, and the num...Figure 4.3. Inclusion of a methane (or ammonia) molecule in a small cage (a) and...Figure 4.4. Geometric characteristics of a CH4–H2O (or NH3–H2O) pair. The positi...Figure 4.5. Contour maps of the potential energy surfaces | (meV) of CH4 trapped...Figure 4.6. Orientational level diagrams of the CH4 molecule trapped in the clat...Figure 4.7. Diagrams of the orientational levels of the NH3 molecule trapped in ...Figure 4.8. Contour maps of the potential energy surface | (meV) of NH3 trapped ...Figure 4.9. Bar spectrum calculated in the vibrational mode 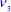 range of CH4 trapp...
range of CH4 trapp...
5 Chapter 5Figure 5.1. Geometry of the fullerene C60 molecule
Читать дальше

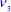 range of CH4 trapp...
range of CH4 trapp...










