Max Diem - Quantum Mechanical Foundations of Molecular Spectroscopy
Здесь есть возможность читать онлайн «Max Diem - Quantum Mechanical Foundations of Molecular Spectroscopy» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Quantum Mechanical Foundations of Molecular Spectroscopy
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:5 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
Quantum Mechanical Foundations of Molecular Spectroscopy: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Quantum Mechanical Foundations of Molecular Spectroscopy»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
covers the quantum mechanical fundamentals of molecular spectroscopy from the view of a professional spectroscopist, rather than a theoretician. Written by a noted expert on the topic, the book puts the emphasis on the relationship between spectroscopy and quantum mechanics, and provides the background information and derivations of the subjects needed to understand spectroscopy including: stationary energy states, transitions between these states, selection rules, and symmetry.
The phenomenal growth of all forms of spectroscopy over the past eight decades has contributed enormously to our understanding of molecular structure and properties. Today spectroscopy covers a broad field including the modern magnetic resonance techniques, non-linear, laser and fiber-based spectroscopy, surface and surface-enhanced spectroscopy, pico- and femtosecond time resolved spectroscopy, and many more. This up-to-date resource discusses several forms of spectroscopy that are used in many fields of science, such as fluorescence, surface spectroscopies, linear and non-linear Raman spectroscopy and spin spectroscopy. This important text:
Contains the physics and mathematics needed to understand spectroscopy Explores spectroscopic methods the are widely used in chemistry, biophysics, biology, and materials science Offers a text written by an experienced lecturer and practitioner of spectroscopic methods Includes detailed explanations and worked examples Written for chemistry, biochemistry, material sciences, and physics students,
provides an accessible text for understanding molecular spectroscopy.

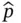 , defined by
, defined by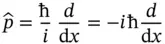
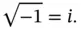 Equation (2.2)often is considered the central postulate of QM.
Equation (2.2)often is considered the central postulate of QM.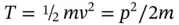
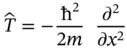
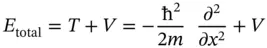
 . This can be written as
. This can be written as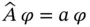
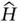 and referred to as the Hamilton operator, or the Hamiltonian, of the system. With the definition of the Hamiltonian above, it is customary to write the total energy equation of the system as
and referred to as the Hamilton operator, or the Hamiltonian, of the system. With the definition of the Hamiltonian above, it is customary to write the total energy equation of the system as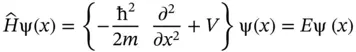
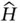 on a set of (still unknown) eigenfunctions ψ that are here assumed to be time‐independent and a function of spatial coordinates x only, ψ( x ). Solving the differential equations given by Eq. (2.7)yields the eigenfunctions ψ iand their associated energy eigenvalues E i.
on a set of (still unknown) eigenfunctions ψ that are here assumed to be time‐independent and a function of spatial coordinates x only, ψ( x ). Solving the differential equations given by Eq. (2.7)yields the eigenfunctions ψ iand their associated energy eigenvalues E i. , for repeated measurements, is given by
, for repeated measurements, is given by