(1.18) 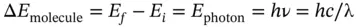
where the subscript f and i denote, respectively, the final and initial (energy) state of the atom (or molecule). Such a process is referred to as a “emission” of a photon. Similarly, an absorption process is one in which the atom undergoes a transition from a lower to a higher energy state, the energy difference being provided by a photon that is annihilated in the process. Absorption and emission processes are collectively referred to as “transitions” between stationary states and are directly related to the annihilation and creation, respectively, of a photon.
The wavelengths or energies from the hydrogen emission or absorption experiments were fit by an empirical equation known as the Rydberg equation, which gave the energy “states” of the hydrogen atom as
(1.19) 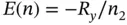
In this equation, n is an integer (>0) “quantum” number, and R yis the Rydberg constant, ( R y= 2.179 × 10 −18J). This equation implies that the energy of the hydrogen atom cannot assume arbitrary energy values, but only “quantized” levels, E ( n ). This observation led to the ideas of electrons in stationary planetary orbits around the nucleus, which – however – was in contradiction with existing knowledge of electrodynamics, as discussed in the beginning of this chapter.
The energy level diagram described by Eq. (1.19)is depicted in Figure 1.4. Here, the sign convention is as follows. For n = ∞, the energy of interaction between nucleus and electron is zero, since the electron is no longer associated with the nucleus. The lowest energy state is given by n = 1, which corresponds to the H atom in its ground state that has a negative energy of 2.179 × 10 −18J.
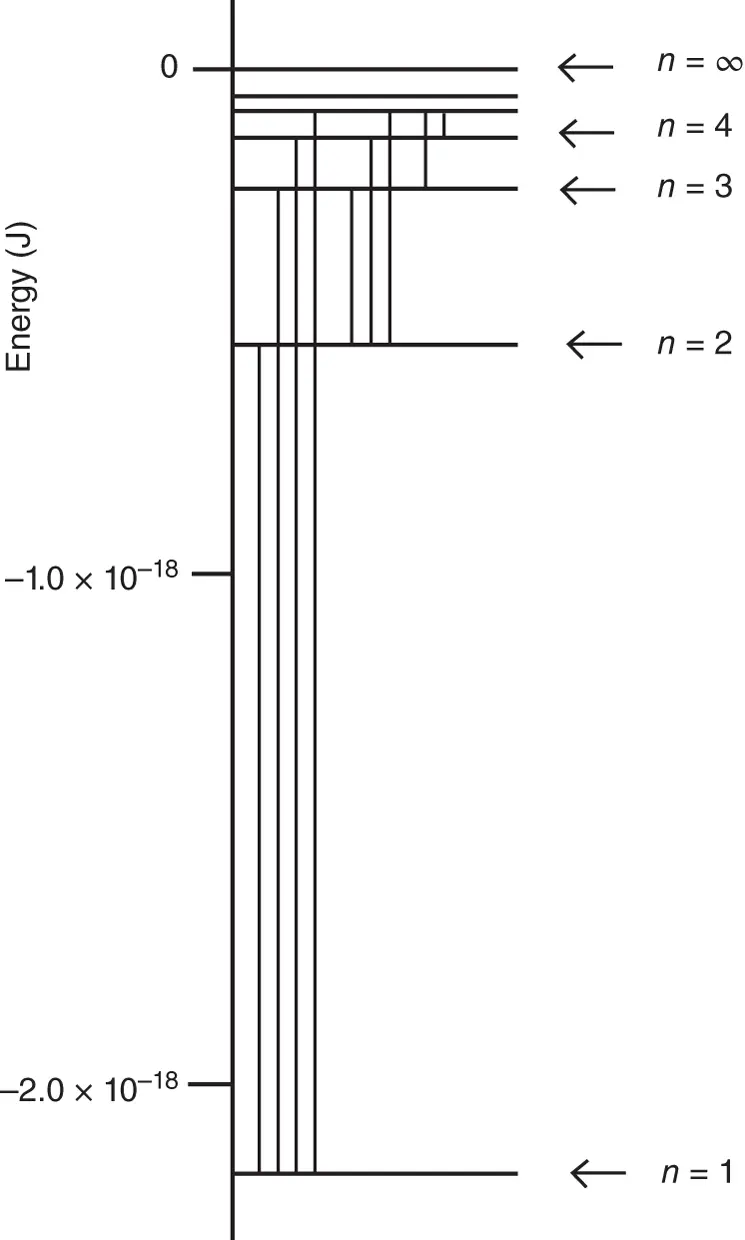
Figure 1.4 Energy level diagram of the hydrogen atom. Transitions between the energy levels are indicated by vertical lines.
Equation (1.19)provided a background framework to explain the hydrogen atom emission spectrum. According to Eq. (1.19), the energy of a photon, or the energy difference of the atomic energy levels, between any two states n fand n ican be written as
(1.20) 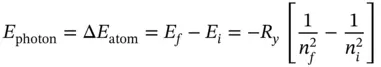
At this point, an example may be appropriate to demonstrate how this empirically derived equation predicts the energy, wavelength, and wavenumber of light emitted by hydrogen atoms. This example also introduces a common problem, namely, that of units. Although there is an international agreement about what units (the système international, or SI units) are to be used to describe spectral transitions, the problem is that few people are using them. In this book, all efforts will be made to use SI units, or at least give the conversion to other units.
The sign conventions used here are similar to those in thermodynamics where a process with a final energy state lower than that of the initial state is called an “exothermic” process, where heat or energy is lost. In Example 1.2, the energy is lost as a photon and is called an emission transition. When describing an absorption process, the energy difference of the atom is negative, Δ E atom< 0, that is, the atom has gained energy (“endothermic” process in thermodynamics). Following the procedure outlined in Example 1.2would lead to a negative wavelength of the photon, which of course is physically meaningless, and one has to remember that the negative Δ E atomimplies the absorption of a photon.
Example 1.2Calculation of the energy, frequency, wavelength, and wavenumber of a photon emitted by a hydrogen atom undergoing a transition from n = 6 to n = 2.
Answer:
The energy difference between the two states of the hydrogen atom is given by
(E1.2.1) 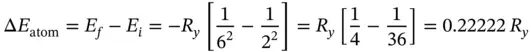
Using the value of the Rydberg constant given above, R y= 2.179 × 10 −18J, the energy difference is
(E1.2.2) 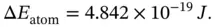
Using Eq. (1.12), Δ E = E photon = hν = hc/λ, the frequency ν is found to be
(E1.2.3) 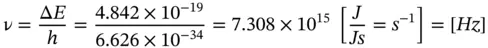
The wavelength of such a photon is given by Eq. (1.7)as
(E1.2.4) 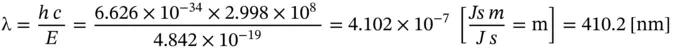
that is, a photon in the ultraviolet wavelength range. Finally, the wavenumber of this photon is
(E1.2.5) 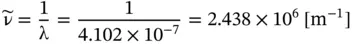
This is a case where the SI units are used infrequently, and the results for the wavenumber are usually given by spectroscopists in units of cm −1, where 1 m −1= 10 −2cm −1. Accordingly, the results in Eq. E1.5 is written as
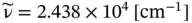 or about 24 380 cm −1.
or about 24 380 cm −1.
1.5 Molecular Spectroscopy
Example 1.2in the previous section describes an emission process in atomic spectroscopy , a subject covered briefly in Chapter 9. Molecular spectroscopy is a branch of science in which the interactions of electromagnetic radiation and molecules are studied, where the molecules exist in quantized stationary energy states similar to those discussed in the previous section. However, these energy states may or may not be due to transitions of electrons into different energy levels, but due to vibrational, rotational, or spin energy levels. Thus, molecular spectroscopy often is classified by the wavelength ranges of the electromagnetic radiation (for example, microwave or infrared spectroscopies) or changes in energy levels of the molecular systems. This is summarized in Table 1.1, and the conversion of wavelengths and energies were discussed in Eqs. (1.11)– (1.15)and are summarized in Appendix 1.
Table 1.1 Photon energies and spectroscopic ranges a .
|
ν photon |
λ photon |
E photon[J] |
E photon[kJ/mol] |
E photon[m −1] |
Transition |
| Radio |
750 MHz |
0.4 m |
5×10 −25 |
3×10 −4 |
2.5 |
NMR b |
| Microwave |
3 GHz |
10 cm |
2×10 −24 |
0.001 |
10 |
EPR b |
| Microwave |
30 GHz |
1 cm |
2×10 −23 |
0.012 |
100 |
Rotational |
| Infrared |
3×10 13Hz |
10 μm |
2×10 −20 |
12 |
10 5 |
Vibrational |
| UV/visible |
10 15 |
300 nm |
6×10 −19 |
360 |
3×10 6 |
Electronic |
| X‐ray |
10 18 |
0.3 nm |
6×10 −16 |
3.6×10 5 |
3×10 9 |
X‐ray absorption |
a) For energy conversions, see Appendix 1.
Читать дальше

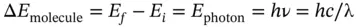
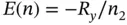

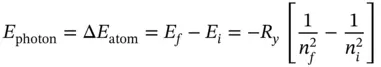
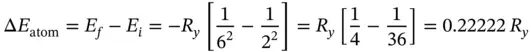
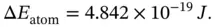
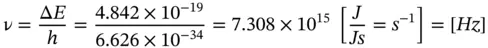
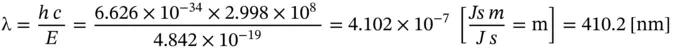
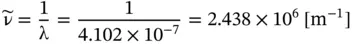
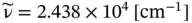 or about 24 380 cm −1.
or about 24 380 cm −1.










