Many of the biological functions of substances are pH-dependent. For this reason, knowing which functional groups are acidic, basic, or neutral is important. Neutral functional groups behave the same no matter what the pH is. Table 3-2lists the functional groups; whether they’re acidic, neutral, or basic; and, for acidic and basic groups, their weakness level (medium, weak, or very weak). The weaker a substance is in terms of pH, the less likely it’s affected by its solution pH (except under extreme pH conditions, which normally don’t occur in biological systems).
TABLE 3-2Acid-Base Properties of Biologically Important Functional Groups
| Functional Group |
Acid, Base, or Neutral |
Weakness Level |
| Monophosphate esters and diphosphate esters |
Acid |
Medium |
| Carboxylic acids |
Acid |
Weak |
| Phenols |
Acid |
Very weak |
| Thiols |
Acid |
Very weak |
| Amine salts |
Acid |
Very weak |
| Amines |
Base |
Weak |
| Carboxylate ions |
Base |
Very weak |
| Alcohols |
Neutral |
|
| Carboxylate esters |
Neutral |
|
| Ethers |
Neutral |
|
| Triphosphate esters |
Neutral |
|
| Thioethers |
Neutral |
|
| Disulfides |
Neutral |
|
| Amides |
Neutral |
|
| Ketones |
Neutral |
|
| Aldehydes |
Neutral |
|
Same Content, Different Structure: Isomerism
 Isomers are compounds that have the same molecular formula but different structural formulas. (It’s all in how things are put together.) Some organic and biochemical compounds may exist in different isomeric forms. Many times, especially in biological systems, these different isomers have different properties. The two most common types of isomers in biological systems are cis-trans isomers and isomerism due to the presence of a chiral carbon. We talk about both of these types in the following sections.
Isomers are compounds that have the same molecular formula but different structural formulas. (It’s all in how things are put together.) Some organic and biochemical compounds may exist in different isomeric forms. Many times, especially in biological systems, these different isomers have different properties. The two most common types of isomers in biological systems are cis-trans isomers and isomerism due to the presence of a chiral carbon. We talk about both of these types in the following sections.
The presence of carbon-carbon double bonds leads to the possibility of having isomers present. Double bonds are rather restrictive and limit molecular movement. Groups on the same side of the double bond tend to remain in that position (cis), whereas groups on opposite sides tend to remain across the bond from each other (trans). See Figure 3-7 for an illustration of cis and trans isomers.

FIGURE 3-7:Cis and trans isomers.
If the two groups attached to either of the carbon atoms of the double bond are the same, cis-trans isomers aren’t possible. Cis isomers are the normal form of fatty acids, whereas food processing tends to convert some of the cis isomers to the trans isomers. The trans isomers are less biologically friendly (as in trans fats, which tend to be carcinogenic) than the cis isomers.
Cis-trans isomers are also possible in cyclic systems. The cis form has similar groups on the same side of the ring, whereas the trans form has similar groups above and below the ring.
Trying to put your gloves on the wrong hands is kind of like another property of biological systems: handedness. Left-handed molecules rotate plane-polarized light to the left, and right-handed molecules rotate plane-polarized light to the right.
Identifying chiral molecules
The presence of an asymmetric, or chiral, carbon atom is sufficient to produce a handed molecule.
 A chiral carbon atom has four different groups attached to it. The majority of biological molecules have one or more chiral carbon atoms and, for this reason, they’re chiral. Figure 3-8 shows the chiral nature of glucose.
A chiral carbon atom has four different groups attached to it. The majority of biological molecules have one or more chiral carbon atoms and, for this reason, they’re chiral. Figure 3-8 shows the chiral nature of glucose.
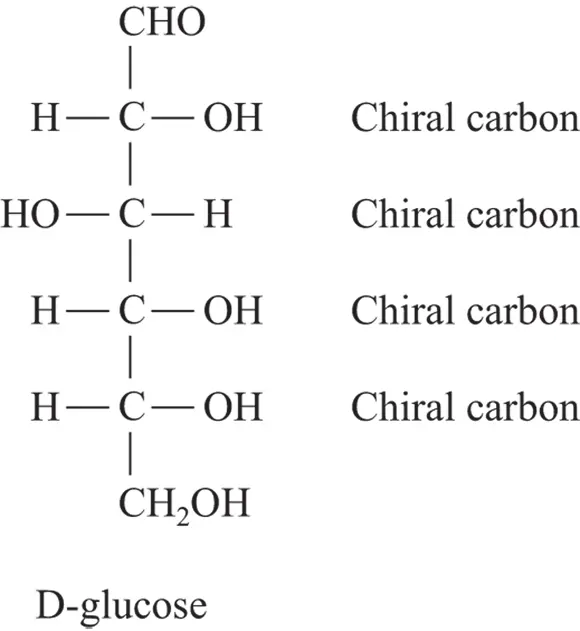
FIGURE 3-8:The structure of glucose, a sugar with four chiral carbon atoms.
Determining the chiral form: Enantiomer or stereoisomer?
All substances have a mirror image (okay, except vampires); however, if a chiral carbon atom is present, the mirror images are nonsuperimposable. Hold out your left and right hands, palms up — they are nonsuperimposable mirror images. These two mirror images are called enantiomers. The different chiral forms differ from each other in two aspects:
How they affect light
How they interact with other chiral substances (usually only one chiral form is biologically active)
To determine how a particular form affects light, it’s necessary to use plane-polarized light, in which all the light waves vibrate in the same plane. When you use this kind of light, a chiral substance rotates the vibrational plane of the light — one form (the dextrorotatory, d , 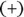 isomer) rotates the plane to the right, and the other (the levorotatory, l ,
isomer) rotates the plane to the right, and the other (the levorotatory, l ,  isomer) rotates the plane to the left. The d and l forms are stereoisomers and are optically active.
isomer) rotates the plane to the left. The d and l forms are stereoisomers and are optically active.
Illustrating the chiral compound: Fischer projection formulas
The chemist Emil Fischer developed a method of drawing a compound to illustrate which stereoisomer was present. These Fischer projection formulas are very useful in biochemistry. In a projection formula, a chiral carbon is placed in the center of a + pattern. The vertical lines (bonds) point away from the viewer, and the horizontal lines point toward the viewer (see Figure 3-9). Fischer used the D designation if the most important group (the group whose central atom had the highest atomic number) was to the right of the carbon, and the L designation if the most important group (again, the group whose central atom has the highest atomic number) was to the left of the carbon. Figure 3-10 shows two Fischer projection formulas.
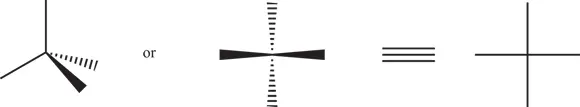
FIGURE 3-9:The construction of a Fischer projection.
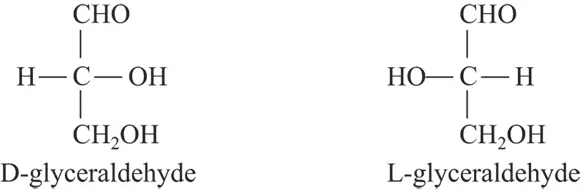
FIGURE 3-10:Fischer projection formulas distinguish stereoisomers.
 The d and l symbols for chiral substance forms aren’t necessarily the Fischer projection formula’s D- and L- forms, respectively; thus, confusion may occur and lead to incorrect predictions. For this reason, the use of d and l is diminishing. The use of D and L is gradually being replaced by the R and S system of designating isomers. This system is particularly useful when more than one chiral carbon atom is present. For a description of this system, see Organic Chemistry For Dummies, by Arthur Winter (Wiley)
The d and l symbols for chiral substance forms aren’t necessarily the Fischer projection formula’s D- and L- forms, respectively; thus, confusion may occur and lead to incorrect predictions. For this reason, the use of d and l is diminishing. The use of D and L is gradually being replaced by the R and S system of designating isomers. This system is particularly useful when more than one chiral carbon atom is present. For a description of this system, see Organic Chemistry For Dummies, by Arthur Winter (Wiley)
Читать дальше

 Isomers are compounds that have the same molecular formula but different structural formulas. (It’s all in how things are put together.) Some organic and biochemical compounds may exist in different isomeric forms. Many times, especially in biological systems, these different isomers have different properties. The two most common types of isomers in biological systems are cis-trans isomers and isomerism due to the presence of a chiral carbon. We talk about both of these types in the following sections.
Isomers are compounds that have the same molecular formula but different structural formulas. (It’s all in how things are put together.) Some organic and biochemical compounds may exist in different isomeric forms. Many times, especially in biological systems, these different isomers have different properties. The two most common types of isomers in biological systems are cis-trans isomers and isomerism due to the presence of a chiral carbon. We talk about both of these types in the following sections.

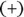 isomer) rotates the plane to the right, and the other (the levorotatory, l ,
isomer) rotates the plane to the right, and the other (the levorotatory, l ,  isomer) rotates the plane to the left. The d and l forms are stereoisomers and are optically active.
isomer) rotates the plane to the left. The d and l forms are stereoisomers and are optically active.
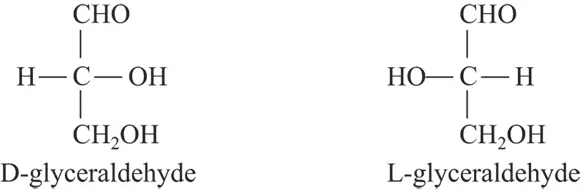
 The d and l symbols for chiral substance forms aren’t necessarily the Fischer projection formula’s D- and L- forms, respectively; thus, confusion may occur and lead to incorrect predictions. For this reason, the use of d and l is diminishing. The use of D and L is gradually being replaced by the R and S system of designating isomers. This system is particularly useful when more than one chiral carbon atom is present. For a description of this system, see Organic Chemistry For Dummies, by Arthur Winter (Wiley)
The d and l symbols for chiral substance forms aren’t necessarily the Fischer projection formula’s D- and L- forms, respectively; thus, confusion may occur and lead to incorrect predictions. For this reason, the use of d and l is diminishing. The use of D and L is gradually being replaced by the R and S system of designating isomers. This system is particularly useful when more than one chiral carbon atom is present. For a description of this system, see Organic Chemistry For Dummies, by Arthur Winter (Wiley)










