John T. Moore - Biochemistry For Dummies
Здесь есть возможность читать онлайн «John T. Moore - Biochemistry For Dummies» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Biochemistry For Dummies
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:5 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
Biochemistry For Dummies: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Biochemistry For Dummies»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
In
you’ll explore the carbons, proteins, and cellular systems that make up the biochemical processes that create and sustain life of all kinds. Perfect for students majoring in biology, chemistry, pre-med, health-services, and other science-related fields, this book tracks a typical college-level biochemistry class. It simplifies and clarifies the subject with easy-to-follow diagrams and real-world examples. You’ll also get:
Explorations of cell biology, carbohydrates, proteins, lipids, and other fundamental building blocks of life Discussions of the basic structures common to all living organisms Treatments of the microscopic details of life that make us all tick If you’re looking for a hand with some of the trickier parts of biochemistry—or you just need an accessible overview of the subject—check out
today!

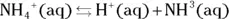
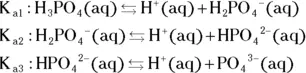
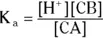
 The value for each successive equilibrium constant often is significantly lower than the preceding value. Table 2-2illustrates some biologically important acids. You can refer to this table when working buffer problems or determining which acid is stronger.
The value for each successive equilibrium constant often is significantly lower than the preceding value. Table 2-2illustrates some biologically important acids. You can refer to this table when working buffer problems or determining which acid is stronger.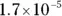
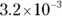
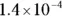
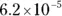
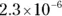
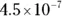
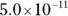
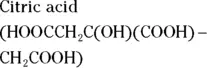
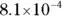
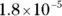
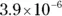
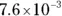
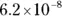
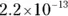
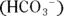 can act as either an acid or a base:
can act as either an acid or a base: group and an acidic carboxyl
group and an acidic carboxyl 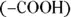 group. Therefore, they can act as either acids or bases. For example, glycine
group. Therefore, they can act as either acids or bases. For example, glycine 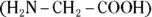 may undergo the following reactions:
may undergo the following reactions: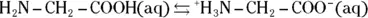
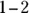 , and the pH in the intestinal tract is
, and the pH in the intestinal tract is 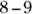 . If the pH of blood is more than 0.2 pH units lower than normal, a condition known as acidosis results; a corresponding increase in pH of about the same magnitude is alkalosis. Acidosis and alkalosis, which may lead to serious health problems, each have two general causes:
. If the pH of blood is more than 0.2 pH units lower than normal, a condition known as acidosis results; a corresponding increase in pH of about the same magnitude is alkalosis. Acidosis and alkalosis, which may lead to serious health problems, each have two general causes:










