 To resist these pH problems, the blood has a number of buffer systems — systems that resist a change in pH by reacting with either added acids or bases. In general, buffers may be amphiprotic substances or mixtures of weak acids and weak bases. In the body, these include several proteins in blood plasma and the bicarbonate buffer system.
To resist these pH problems, the blood has a number of buffer systems — systems that resist a change in pH by reacting with either added acids or bases. In general, buffers may be amphiprotic substances or mixtures of weak acids and weak bases. In the body, these include several proteins in blood plasma and the bicarbonate buffer system.
The bicarbonate buffer system is the main extracellular buffer system. This system also provides a means of eliminating carbon dioxide. The dissolution of carbon dioxide in aqueous systems sets up the following equilibrium:
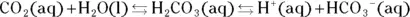
The presence of the conjugate acid-base pair (H 2CO 3and 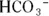 ) means that this is a buffer system. The conjugate acid-base ratio is about 20:1 at a pH of 7.4 in the bloodstream. This buffer system is coupled with the following equilibrium (instrumental in the removal of carbon dioxide in the lungs):
) means that this is a buffer system. The conjugate acid-base ratio is about 20:1 at a pH of 7.4 in the bloodstream. This buffer system is coupled with the following equilibrium (instrumental in the removal of carbon dioxide in the lungs):
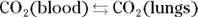
The second ionization of phosphoric acid, K a2, is the primary intracellular buffer system. The pH of this conjugate acid-base pair ( 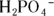 and
and 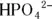 ) is 7.21 for a solution with equal concentrations of these two species.
) is 7.21 for a solution with equal concentrations of these two species.
Calculating a buffer’s pH
To determine a buffer’s pH, you can use a K aor K bcalculation, as we discuss in the section “ Swapping hydrogens between acids and bases,” earlier in this chapter, or you can use the Henderson-Hasselbalch equation, which is a shortcut.
The Henderson-Hasselbalch equation takes two forms:
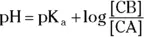
and
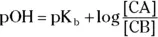
The terms in either form are the same as those we define earlier in the chapter. For example, suppose you want to calculate the pH of a buffer composed of 0.15 M pyruvic acid and 0.25 M sodium pyruvate. Referring back to Table 2-2, you see that the K aof pyruvic acid is 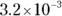 .
.
The pK awould be 2.50. Therefore:
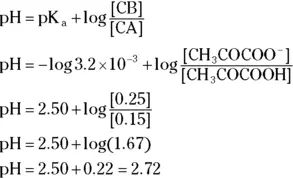
 The greater the values of [CA] and [CB], the greater the buffer capacity of the solution. The buffer capacity indicates how much acid or base may be added to a buffer before the buffer ceases to function as a buffer. A buffer in which the
The greater the values of [CA] and [CB], the greater the buffer capacity of the solution. The buffer capacity indicates how much acid or base may be added to a buffer before the buffer ceases to function as a buffer. A buffer in which the 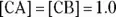 would have a much higher buffer capacity for adding either acids or bases than a buffer in which the
would have a much higher buffer capacity for adding either acids or bases than a buffer in which the 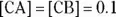 . If there were a buffer in which
. If there were a buffer in which 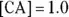 and its
and its 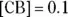 , the buffer would have a higher buffer capacity for additions of a base than for additions of an acid because the buffer contains more acid than base. For the buffer to be as flexible as possible, the concentrations of the conjugate acid-base pair should be as close to equal as possible and as high as possible.
, the buffer would have a higher buffer capacity for additions of a base than for additions of an acid because the buffer contains more acid than base. For the buffer to be as flexible as possible, the concentrations of the conjugate acid-base pair should be as close to equal as possible and as high as possible.
Chapter 3
Fun with Carbon: Organic Chemistry
IN THIS CHAPTER
 Understanding why carbon is fundamental to biochemistry
Understanding why carbon is fundamental to biochemistry
 Measuring the strength of different kinds of bonds
Measuring the strength of different kinds of bonds
 Finding out about functional groups
Finding out about functional groups
 Checking out isomerism
Checking out isomerism
Most biologically important molecules are composed of organic compounds, compounds of carbon. Therefore, you, as a student of biochemistry, must have a general knowledge of organic chemistry, which is the study of carbon compounds, in order to understand the functions and reactions of biochemical molecules. In this chapter, we go over the basics of organic chemistry, including the various functional groups and isomers that are important in the field of biochemistry. (We’re sure that this chapter will bring back fond memories of your organic chemistry classes and labs.)
If you feel you need a little more background in organic chemistry, refer to Organic Chemistry I For Dummies (written by Arthur Winter and published by Wiley); Organic Chemistry II For Dummies, by these two wonderful authors (Wiley); and even Chemistry For Dummies, by John (Wiley). (Shameless plugs for others of our books!)
The Role of Carbon in the Study of Life
Long ago, scientists believed that all carbon compounds were the result of biological processes, which meant that organic chemistry was synonymous with biochemistry under what was known as the Vital Force theory. In the mid-1800s, though, researchers such as Friedrich Wöhler debunked that long-held notion; the synthesis of urea, CO(NH 2) 2, from an inorganic material (ammonium cyanate, NH 4OCN) showed that other paths to the production of carbon compounds existed. Organic chemists now synthesize many important organic chemicals without the use of living organisms; however, biosynthesis is still an important source of many organic compounds.
Why are there so many carbon compounds? The answer lies primarily in two reasons, both tied to carbon’s versatility in creating stable bonds:
Carbon bonds to itself. Carbon atoms are capable of forming stable bonds to other carbon atoms. The process of one type of atom bonding to identical atoms is catenation. Many other elements can catenate, but carbon is the most efficient at it. There appears to be no limit to how many carbon atoms can link together. These linkages may be in chains, branched chains, or rings, as shown in Figure 3-1.
Читать дальше

 To resist these pH problems, the blood has a number of buffer systems — systems that resist a change in pH by reacting with either added acids or bases. In general, buffers may be amphiprotic substances or mixtures of weak acids and weak bases. In the body, these include several proteins in blood plasma and the bicarbonate buffer system.
To resist these pH problems, the blood has a number of buffer systems — systems that resist a change in pH by reacting with either added acids or bases. In general, buffers may be amphiprotic substances or mixtures of weak acids and weak bases. In the body, these include several proteins in blood plasma and the bicarbonate buffer system.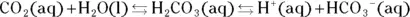
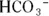 ) means that this is a buffer system. The conjugate acid-base ratio is about 20:1 at a pH of 7.4 in the bloodstream. This buffer system is coupled with the following equilibrium (instrumental in the removal of carbon dioxide in the lungs):
) means that this is a buffer system. The conjugate acid-base ratio is about 20:1 at a pH of 7.4 in the bloodstream. This buffer system is coupled with the following equilibrium (instrumental in the removal of carbon dioxide in the lungs):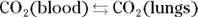
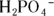 and
and 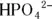 ) is 7.21 for a solution with equal concentrations of these two species.
) is 7.21 for a solution with equal concentrations of these two species.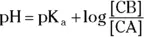
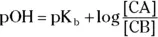
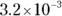 .
.
 The greater the values of [CA] and [CB], the greater the buffer capacity of the solution. The buffer capacity indicates how much acid or base may be added to a buffer before the buffer ceases to function as a buffer. A buffer in which the
The greater the values of [CA] and [CB], the greater the buffer capacity of the solution. The buffer capacity indicates how much acid or base may be added to a buffer before the buffer ceases to function as a buffer. A buffer in which the 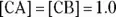 would have a much higher buffer capacity for adding either acids or bases than a buffer in which the
would have a much higher buffer capacity for adding either acids or bases than a buffer in which the 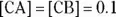 . If there were a buffer in which
. If there were a buffer in which 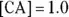 and its
and its 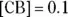 , the buffer would have a higher buffer capacity for additions of a base than for additions of an acid because the buffer contains more acid than base. For the buffer to be as flexible as possible, the concentrations of the conjugate acid-base pair should be as close to equal as possible and as high as possible.
, the buffer would have a higher buffer capacity for additions of a base than for additions of an acid because the buffer contains more acid than base. For the buffer to be as flexible as possible, the concentrations of the conjugate acid-base pair should be as close to equal as possible and as high as possible. Understanding why carbon is fundamental to biochemistry
Understanding why carbon is fundamental to biochemistry










