Carbon bonds to other elements. Carbon is capable of forming stable bonds to a number of other elements. These include the biochemically important elements hydrogen, nitrogen, oxygen, and sulfur. The latter three elements form the foundation of most of the functional groups (reactive groups of a molecule) necessary for life. Bonds between carbon and hydrogen are usually unreactive under biochemical conditions; thus, hydrogen often serves as an inert substituent (an atom or group of atoms taking the place of another atom or group or occupying a specified position in a molecule).
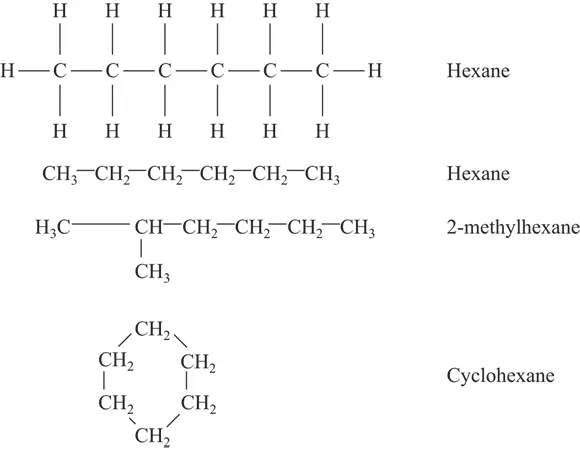
FIGURE 3-1:Top: straight chain hydrocarbon expanded and condensed. Middle: branched chain hydrocarbon. Bottom: ring hydrocarbon.
It’s All in the Numbers: Carbon Bonds
 Carbon is capable of forming four bonds. In bonding to itself and other elements, carbon uses a variety of types of hybridization. When it bonds to other atoms, for example, possible hybridizations include four single bonds, one double and two single bonds, two double bonds, or a triple and a single bond. Double bonds to oxygen atoms are particularly important in many biochemicals. Table 3-1shows the number of bonds carbon may form with some selected nonmetals, along with the hybridization of those bonds.
Carbon is capable of forming four bonds. In bonding to itself and other elements, carbon uses a variety of types of hybridization. When it bonds to other atoms, for example, possible hybridizations include four single bonds, one double and two single bonds, two double bonds, or a triple and a single bond. Double bonds to oxygen atoms are particularly important in many biochemicals. Table 3-1shows the number of bonds carbon may form with some selected nonmetals, along with the hybridization of those bonds.
TABLE 3-1Possible Bonds of Carbon and Selected Nonmetals
| Element |
Number of Possible Bonds with Carbon |
Some Possible Hybridizations for Second Period Elements |
| Carbon (C) |
4 |
4 single (sp 3); 2 single and 1 double (sp 2); 1 single and 1 triple (sp); 2 double (sp) |
| Nitrogen (N) |
3 |
3 single (sp 3); 1 single and 1 double (sp 2); 1 triple (sp) |
| Oxygen (O) |
2 |
2 single (sp 3); 1 double (sp 2) |
| Sulfur (S) |
2 |
2 single (sp 3); 1 double (sp 2) |
| Hydrogen (H) |
1 |
1 single |
| Fluorine (F) |
1 |
1 single |
| Chlorine (Cl) |
1 |
1 single |
| Bromine (Br) |
1 |
1 single |
| Iodine (I) |
1 |
1 single |
When Forces Attract: Bond Strengths
Covalent bonds are important intramolecular forces (forces within the same molecule) in biochemistry. Intermolecular forces (forces between chemical species) are also extremely important. Among other things, intermolecular forces are important to hydrophilic (water-loving) and hydrophobic (water-hating) interactions. ( Phobias involve fear or hate, so hydrophobic is water-hating.)
Everybody has ‘em: Intermolecular forces
All intermolecular forces are van der Waals forces — that is, they’re not true bonds in the sense of sharing or transferring electrons but are weaker attractive forces. These forces include London dispersion forces, dipole-dipole forces, hydrogen bonding, and ionic interactions, all of which we discuss in the following sections.
London dispersion forces are very weak and short-lived attractions between molecules that arise from the nucleus of one atom attracting the electron cloud of another atom. These forces are normally only significant when other intermolecular forces are not present, as in nonpolar molecules.
Dipole-dipole forces exist between polar regions of different molecules. The presence of a dipole means that the molecule has a partially positive 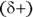 end and a partially negative
end and a partially negative 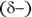 end. Oppositely charged partial charges attract each other, whereas like partial charges repel. In most cases, biological systems utilize a special type of dipole-dipole force known as hydrogen bonding (see the following section). (We said, “hydrogen bond,” not “hydrogen bomb” — there’s a big difference!)
end. Oppositely charged partial charges attract each other, whereas like partial charges repel. In most cases, biological systems utilize a special type of dipole-dipole force known as hydrogen bonding (see the following section). (We said, “hydrogen bond,” not “hydrogen bomb” — there’s a big difference!)
Hydrogen bonding, as the name implies, involves hydrogen. The hydrogen atom must be bonded to either an oxygen atom or a nitrogen atom. (In nonbiological situations, hydrogen bonding also occurs when a hydrogen atom bonds to a fluorine atom or sometimes a chlorine atom.) Hydrogen bonding is significantly stronger than a normal dipole-dipole force and is much stronger than London dispersion forces (discussed in the preceding sections). The hydrogen that bonds to either a nitrogen or an oxygen atom is strongly attracted to a different nitrogen or oxygen atom. Hydrogen bonding may be either intramolecular or intermolecular.
In biological systems, ionic interactions, in which oppositely charged ions attract each other strongly, may serve as intermolecular or intramolecular forces. In some cases, these may involve metal cations, such as 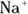 , or anions, such as
, or anions, such as 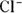 . In many cases, the cation is an ammonium ion from an amino group, such as
. In many cases, the cation is an ammonium ion from an amino group, such as  ; the anion may be from a carboxylic acid, such as
; the anion may be from a carboxylic acid, such as 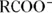 .
.
In nonbiological systems, such interactions are commonly referred to as ionic bonding.
Water-related interactions: Both the lovers and the haters
The predominant factor leading to hydrophobic interactions is the presence of portions of a molecule containing only carbon and hydrogen. Hydrocarbon regions are nonpolar and are attracted to other nonpolar regions by London dispersion forces.
 In general, the presence of any atom other than carbon and hydrogen makes a region polar. Oxygen and nitrogen are the most effective elements in biochemistry for making a region of a molecule polar. Sulfur is the least effective of the common biologically important elements at imparting polar character. Dipole-dipole, hydrogen bonding, and ionic interactions are all hydrophilic interactions. London dispersion forces are hydrophobic interactions. (See the section “ Everybody has ‘em: Intermolecular forces,” earlier in this chapter, for the lowdown on these types of interactions.)
In general, the presence of any atom other than carbon and hydrogen makes a region polar. Oxygen and nitrogen are the most effective elements in biochemistry for making a region of a molecule polar. Sulfur is the least effective of the common biologically important elements at imparting polar character. Dipole-dipole, hydrogen bonding, and ionic interactions are all hydrophilic interactions. London dispersion forces are hydrophobic interactions. (See the section “ Everybody has ‘em: Intermolecular forces,” earlier in this chapter, for the lowdown on these types of interactions.)
 The more carbon and hydrogen atoms that are present without other atoms, the more important the hydrophobic nature of a region becomes in defining the molecule’s properties. A molecule may have both a hydrophilic and a hydrophobic region, and both regions are important to the molecule’s behavior. The formation of a micelle (see Chapter 2) is an example of using molecules with both hydrophilic and hydrophobic regions. Think of these micelles every time you wash dishes. The soap or detergent dissolves the grease or oil and forms a micelle, keeping the grease in solution so that it can go down the drain.
The more carbon and hydrogen atoms that are present without other atoms, the more important the hydrophobic nature of a region becomes in defining the molecule’s properties. A molecule may have both a hydrophilic and a hydrophobic region, and both regions are important to the molecule’s behavior. The formation of a micelle (see Chapter 2) is an example of using molecules with both hydrophilic and hydrophobic regions. Think of these micelles every time you wash dishes. The soap or detergent dissolves the grease or oil and forms a micelle, keeping the grease in solution so that it can go down the drain.
Читать дальше


 Carbon is capable of forming four bonds. In bonding to itself and other elements, carbon uses a variety of types of hybridization. When it bonds to other atoms, for example, possible hybridizations include four single bonds, one double and two single bonds, two double bonds, or a triple and a single bond. Double bonds to oxygen atoms are particularly important in many biochemicals. Table 3-1shows the number of bonds carbon may form with some selected nonmetals, along with the hybridization of those bonds.
Carbon is capable of forming four bonds. In bonding to itself and other elements, carbon uses a variety of types of hybridization. When it bonds to other atoms, for example, possible hybridizations include four single bonds, one double and two single bonds, two double bonds, or a triple and a single bond. Double bonds to oxygen atoms are particularly important in many biochemicals. Table 3-1shows the number of bonds carbon may form with some selected nonmetals, along with the hybridization of those bonds.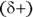 end and a partially negative
end and a partially negative 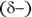 end. Oppositely charged partial charges attract each other, whereas like partial charges repel. In most cases, biological systems utilize a special type of dipole-dipole force known as hydrogen bonding (see the following section). (We said, “hydrogen bond,” not “hydrogen bomb” — there’s a big difference!)
end. Oppositely charged partial charges attract each other, whereas like partial charges repel. In most cases, biological systems utilize a special type of dipole-dipole force known as hydrogen bonding (see the following section). (We said, “hydrogen bond,” not “hydrogen bomb” — there’s a big difference!)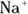 , or anions, such as
, or anions, such as 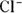 . In many cases, the cation is an ammonium ion from an amino group, such as
. In many cases, the cation is an ammonium ion from an amino group, such as  ; the anion may be from a carboxylic acid, such as
; the anion may be from a carboxylic acid, such as 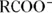 .
.










