How bond strengths affect physical properties of substances
The physical properties of biological substances depend on the intermolecular forces present. The general order of strength is:
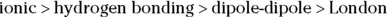
A few exceptions occur in borderline cases (molecules that are not simple molecular species) and for very large molecules (called macromolecules ).
The strongest types of intermolecular forces involve ions. Next strongest is hydrogen bonding. Polar substances interact through dipole-dipole forces, which are weaker than hydrogen bonds. All biological substances containing oxygen, nitrogen, sulfur, or phosphorus are polar. London forces, the weakest intermolecular forces, are important in nonpolar situations. The hydrocarbon portion of biological molecules is nonpolar.
Melting points, boiling points, and solubility
As the strength of forces decreases, so do the melting points, boiling points, and solubility in water. In addition, the vapor pressure and the solubility in nonpolar solvents increase.
 Substances that have a high solubility in water are hydrophilic, and substances that have a low solubility in water are hydrophobic. You can get the scoop on solubility in the section.
Substances that have a high solubility in water are hydrophilic, and substances that have a low solubility in water are hydrophobic. You can get the scoop on solubility in the section.
A molecule may have both hydrophilic and hydrophobic regions. The region that represents a greater portion of the molecule predominates. For this reason, for example,
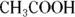
is more hydrophilic than
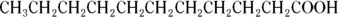
because the hydrophilic end (-COOH) is a much more significant portion of the entire molecule in the first case than in the second case.
In addition,
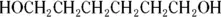
is more hydrophilic than
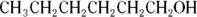
because of the presence of the second hydrophilic region 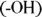 .
.
 Many functional groups have distinctive odors. Small carboxylic acids smell like acetic acid (vinegar), while larger ones have unpleasant odors. Most esters, if volatile, have pleasant odors, which is why esters are used extensively in the food and perfume industry. Most sulfur-containing compounds have strong, unpleasant odors. Small amines have an ammonia odor, whereas larger amines have a fishy odor or worse. That’s why people squeeze lemon juice on fish — the acidic lemon juice reacts with the basic amines to form an ammonium salt that doesn’t have an odor. (Believe us, there are odors worse than fish — putrescine is one!)
Many functional groups have distinctive odors. Small carboxylic acids smell like acetic acid (vinegar), while larger ones have unpleasant odors. Most esters, if volatile, have pleasant odors, which is why esters are used extensively in the food and perfume industry. Most sulfur-containing compounds have strong, unpleasant odors. Small amines have an ammonia odor, whereas larger amines have a fishy odor or worse. That’s why people squeeze lemon juice on fish — the acidic lemon juice reacts with the basic amines to form an ammonium salt that doesn’t have an odor. (Believe us, there are odors worse than fish — putrescine is one!)
Getting a Reaction out of a Molecule: Functional Groups
Most carbon compounds have one or more reactive sites composed of a specific grouping of atoms in their structure. These sites are where chemical reactions occur. These specific groupings of atoms that react are called functional groups. Functional groups contain atoms other than carbon and hydrogen and/or double or triple bonds, and they define the reactivity of the organic molecule.
Alkanes are hydrocarbons — compounds containing only carbon and hydrogen, with no traditional functional groups. For this reason, they aren’t very reactive. Alkenes and alkynes are also hydrocarbons. They contain carbon-carbon double and triple bonds, respectively. The presence of more than one type of bond makes them more reactive. Aromatic hydrocarbons, normally ring structures with alternating single and double carbon-to-carbon bonds, contain one or more aromatic systems, which are much less reactive than other systems containing double bonds. Alkynes aren’t very common in biological systems. Figure 3-2 shows the structure of these compounds.
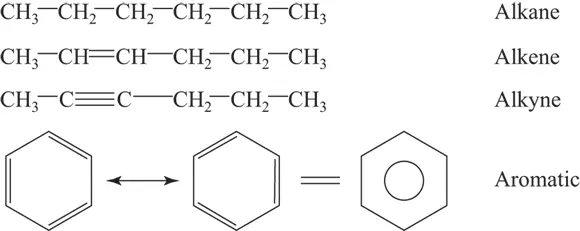
FIGURE 3-2:Examples of alkanes, alkenes, alkynes, and aromatic hydrocarbons.
Functional groups with oxygen and sulfur
Many functional groups contain oxygen, including alcohols, ethers, aldehydes, and ketones. You encounter many of these oxygen-containing functional groups when you study carbohydrates (one of our favorite things). In carbohydrates, many times the ether groups are referred to as glycoside linkages (more on carbohydrates in Chapter 7) . In addition, carboxylic acids and esters are important functional groups that appear as fatty acids and in fats and oils.
Alcohols and ethers contain only singly bonded oxygen atoms. An alcohol group attached to an aromatic ring is a phenol. Aldehyde and ketone functional groups contain only doubly bonded oxygen atoms. Carboxylic acids and esters contain both singly and doubly bonded oxygen atoms. The combination of a carbon atom connected to an oxygen atom by a double bond is a carbonyl group.
Sulfur, the element immediately below oxygen on the periodic table, may replace oxygen in both alcohols and ethers to give thiols (mercaptans) and thioethers. Many of these sulfur-containing compounds really stink! Sulfur may also form a disulfide, which has a bond between two sulfur atoms. Figure 3-3 illustrates these compounds.
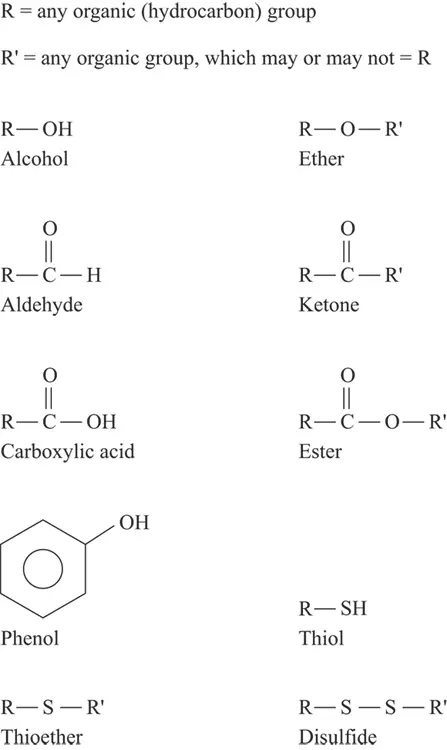
FIGURE 3-3:Oxygen- and sulfur-containing functional groups.
Functional groups containing nitrogen
Amines and amides are two important functional groups containing nitrogen. Amines are present in amino acids and alkaloids. Amides are present in proteins, in which they’re known as peptide bonds.
The difference between amines and amides is that amides have a carbonyl group adjacent to the nitrogen atom. Amines are derivatives of ammonia (NH 3) where one or more organic groups replace hydrogen atoms. In a primary amine, an organic group replaces one hydrogen atom. In secondary and tertiary amines, two or three organic groups, respectively, replace two or three hydrogen atoms. Figure 3-4 shows these compounds, as well as aniline and ammonia.
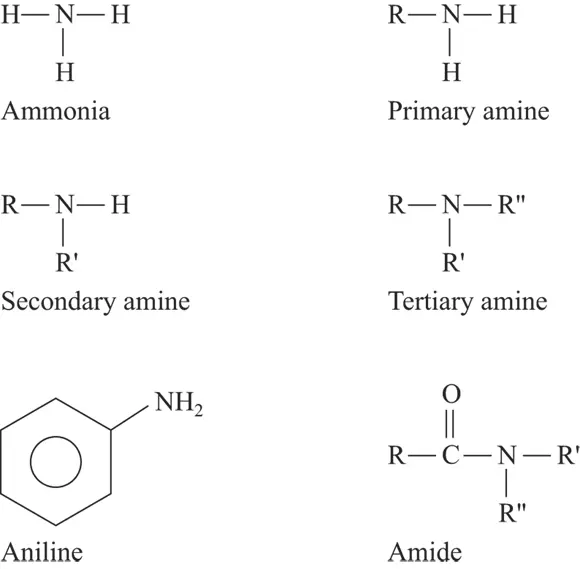
FIGURE 3-4:Some nitrogen-containing functional groups.
Читать дальше

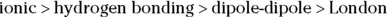
 Substances that have a high solubility in water are hydrophilic, and substances that have a low solubility in water are hydrophobic. You can get the scoop on solubility in the section.
Substances that have a high solubility in water are hydrophilic, and substances that have a low solubility in water are hydrophobic. You can get the scoop on solubility in the section.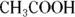
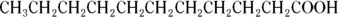
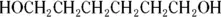
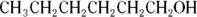
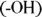 .
. Many functional groups have distinctive odors. Small carboxylic acids smell like acetic acid (vinegar), while larger ones have unpleasant odors. Most esters, if volatile, have pleasant odors, which is why esters are used extensively in the food and perfume industry. Most sulfur-containing compounds have strong, unpleasant odors. Small amines have an ammonia odor, whereas larger amines have a fishy odor or worse. That’s why people squeeze lemon juice on fish — the acidic lemon juice reacts with the basic amines to form an ammonium salt that doesn’t have an odor. (Believe us, there are odors worse than fish — putrescine is one!)
Many functional groups have distinctive odors. Small carboxylic acids smell like acetic acid (vinegar), while larger ones have unpleasant odors. Most esters, if volatile, have pleasant odors, which is why esters are used extensively in the food and perfume industry. Most sulfur-containing compounds have strong, unpleasant odors. Small amines have an ammonia odor, whereas larger amines have a fishy odor or worse. That’s why people squeeze lemon juice on fish — the acidic lemon juice reacts with the basic amines to form an ammonium salt that doesn’t have an odor. (Believe us, there are odors worse than fish — putrescine is one!)













