24.Choukroun J, Adda F, Schoeffler C, Vervelle A. Une opportunité en paro-implantologie: Le PRF. Implantodontie 2001;42:e62.
25.Dohan Ehrenfest DM, Del Corso M, Diss A, Mouhyi J, Charrier JB. Three-dimensional architecture and cell composition of a Choukroun’s platelet-rich fibrin clot and membrane. J Periodontol 2010;81:546–555.
26.Choukroun J, Diss A, Simonpieri A, et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part IV: Clinical effects on tissue healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006;101:e56–e60.
27.Dohan DM, Choukroun J, Diss A, et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006;101:e37–e44.
28.Dohan DM, Choukroun J, Diss A, et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part II: Platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006;101:e45–e50.
29.Martin P, Leibovich SJ. Inflammatory cells during wound repair: The good, the bad and the ugly. Trends Cell Biol 2005;15:599–607.
30.Tsirogianni AK, Moutsopoulos NM, Moutsopoulos HM. Wound healing: Immunological aspects. Injury 2006;37(suppl 1):S5–S12.
31.Adamson R. Role of macrophages in normal wound healing: An overview. J Wound Care 2009;18:349–351.
32.Davis VL, Abukabda AB, Radio NM, et al. Platelet-rich preparations to improve healing. Part I: Workable options for every size practice. J Oral Implantol 2014;40:500–510.
33.Davis VL, Abukabda AB, Radio NM, et al. Platelet-rich preparations to improve healing. Part II: Platelet activation and enrichment, leukocyte inclusion, and other selection criteria. J Oral Implantol 2014;40:511–521.
34.Ghasemzadeh M, Hosseini E. Intravascular leukocyte migration through platelet thrombi: Directing leukocytes to sites of vascular injury. Thromb Haemost 2015;113:1224–1235.
35.Batoon L, Millard SM, Raggatt LJ, Pettit AR. Osteomacs and bone regeneration. Curr Osteoporos Rep 2017;15:385–395.
36.Chang MK, Raggatt LJ, Alexander KA, et al. Osteal tissue macrophages are intercalated throughout human and mouse bone lining tissues and regulate osteoblast function in vitro and in vivo. J Immunol 2008;181:1232–1244.
37.Winkler IG, Sims NA, Pettit AR, et al. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood 2010;116:4815–4828.
38.Dohan DM, Choukroun J, Diss A, et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part III: Leucocyte activation: A new feature for platelet concentrates? Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006;101:e51–e55.
39.Ghanaati S, Booms P, Orlowska A, et al. Advanced platelet-rich fibrin: A new concept for cell-based tissue engineering by means of inflammatory cells. J Oral Implantol 2014;40:679–689.
40.Davies C, Miron RJ. PRF in Facial Esthetics. Chicago: Quintessence, 2020.
41.Miron RJ, Chai J, Zheng S, Feng M, Sculean A, Zhang Y. A novel method for evaluating and quantifying cell types in platelet rich fibrin and an introduction to horizontal centrifugation. J Biomed Mater Res A 2019;107:2257–2271.
42.Varela HA, Souza JCM, Nascimento RM, et al. Injectable platelet rich fibrin: Cell content, morphological, and protein characterization. Clin Oral Investig 2019;23:1309–1318.
2
Biology of PRF: Fibrin Matrix, Growth Factor Release, and Cellular Activity
Contributors
Masako Fujioka-Kobayashi
Yufeng Zhang
Reinhard Gruber
Richard J. Miron
Chapter Highlights
What is PRF?
How does PRF differ from PRP at the biologic and cellular level?
What is the role of each cell type found in PRF?
What is the role of each GF found in PRF?
How does centrifugation speed and time affect PRF?
What advantages exist for horizontal centrifugation versus fixed-angle centrifugation?

 Video 2-1
Video 2-1
Much can be discussed with respect to the biology of PRF and its ability to impact tissue regeneration. During the natural wound healing process, vascularization of tissues plays a pivotal role, facilitating the invasion of incoming cells, growth factors (GFs), cytokines, and other regenerative factors. The main aim of platelet concentrates, discovered over two decades ago, is to favor new blood flow (angiogenesis) to damaged tissues, thereby improving their healing potential by delivering a supraphysiologic concentration of blood-derived cells (namely platelets) and regenerative GFs. This chapter takes a deep look into the actual separation of blood layers during the centrifugation process to provide the clinician a general overview of the cell types and GFs found in PRF, including their roles, and also discusses the effects of centrifugation speed and time on cell layer separation. Furthermore, the advantages of producing an autologous fibrin scaffold are presented as it being a key regulator of wound healing because of its autologous source and its ability to promote the slow and gradual release of GFs over time. The advantages of horizontal centrifugation versus fixed-angle centrifugation are also discussed based on recent data from various laboratories from around the world.
The wound healing process is divided into three stages: the inflammatory phase, the proliferative phase, and the remodeling phase ( Fig 2-1). The inflammatory phase starts at the time of injury and generally involves a wide array of cytokines and growth factors (GFs) that are released within the first 24 to 48 hours. Accordingly, a dynamic interaction occurs between endothelial cells, angiogenic cytokines, and the extracellular matrix (ECM) in an attempt to accelerate wound healing via an orchestrated delivery of multiple GFs in a well-controlled fashion. 1
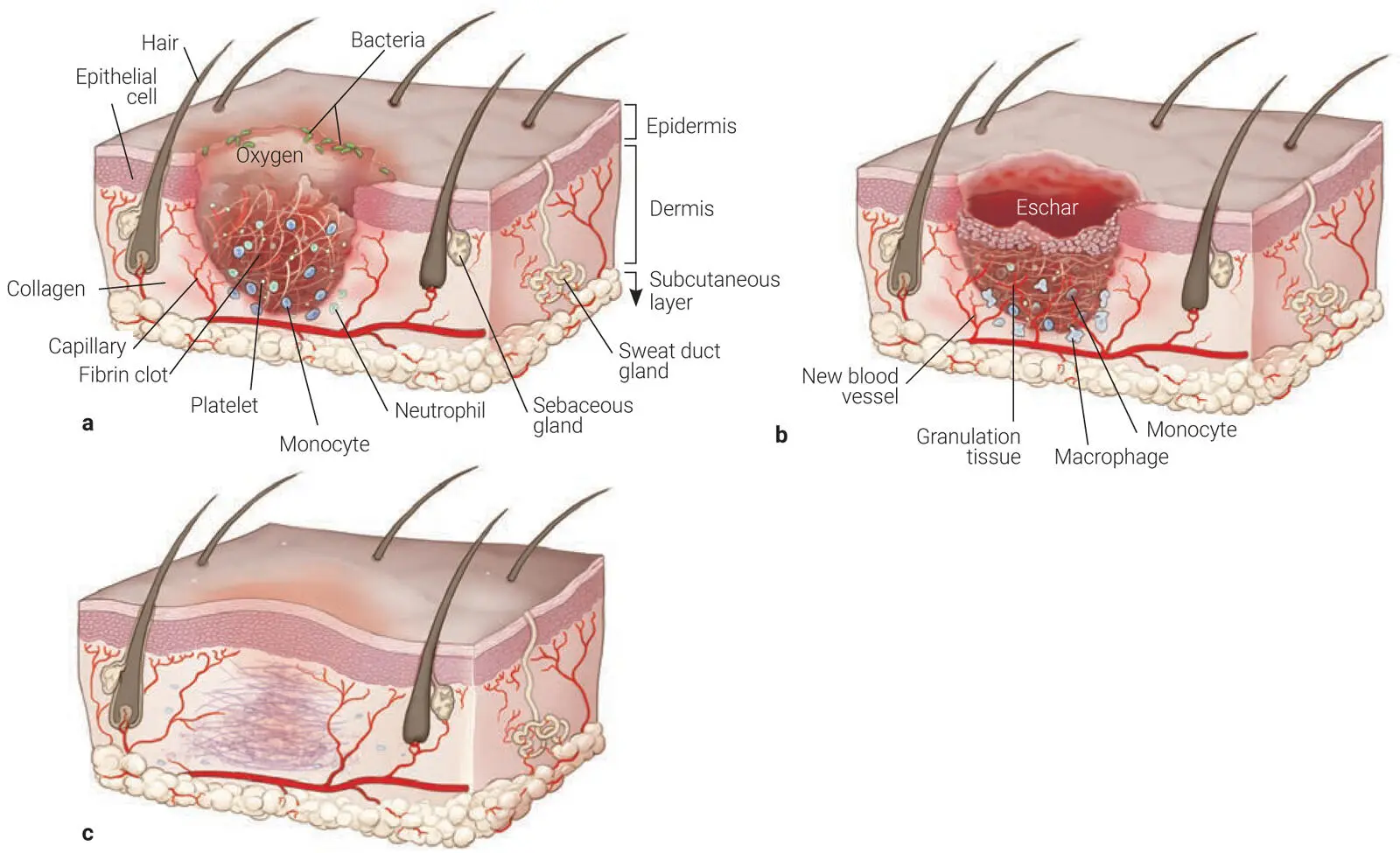
Fig 2-1The three stages of wound healing: (a) Inflammatory phase. (b) Proliferative phase. (c) Remodeling phase.
In general, blood provides essential components to the healing process that comprise both cellular and protein products that essentially are the base components of wound healing. During the healing process, blood will undergo clotting within a few minutes to prevent further blood loss. This is an important step that will be later discussed in the PRF tube section, because in order for clotting to occur and even be improved in both speed and quality (in particular in patients taking anticoagulants), a proper understanding of the clotting cascade is required. In its simplest of forms, oxygen helps improve blood clotting, and for this reason, the simple removal of centrifugation tube lids following the spin process will lead to faster clotting of PRF and a superior fibrin mesh. One of the major roles of platelets is to assist during hemostasis through a fibrin clot formation. 1,2Not surprisingly, the additional use of PRF for wound healing (for example, following tooth removal and extraction socket healing) in patients taking anticoagulants can drastically improve the healing outcomes simply by improving clotting. Because PRF contains many platelets and a fibrin nucleus is already formed, bleeding has been shown to be significantly reduced postoperative when PRF is utilized in patients on anticoagulant therapy. 3
Tips
Oxygen helps improve blood clotting, so the simple removal of centrifugation tube lids following the spin process will lead to faster clotting of PRF.
Читать дальше

 Video 2-1
Video 2-1











