1 ...8 9 10 12 13 14 ...25 
Fig 2-3GF release from PRP and PRF at each time point for PDGF-AA, PDGF-AB, and TGF-β1 over a 10-day period. Notice that while PRP has significantly higher GF release at early time points, over a 10-day period, significantly higher levels are most commonly found with A-PRF due to the slow and gradual release of GFs following use of slower centrifugation speeds. (Adapted from Kobayashi et al. 12)
Overall, PRP can be recommended for fast delivery of GFs, whereas PRF is better suited for long-term delivery and favors better overall wound healing potential.
The Low-Speed Centrifugation Concept
The evolution of PRF in the years 2014 to 2017 was highly focused on ways to improve centrifugation parameters in order to favor more GF release. Within the original L-PRF matrix, cells were surprisingly found gathered at the bottom of the PRF matrix or bottom of centrifugation tubes. 6In simple terms, the faster and longer centrifugation is carried out, the more matter (ie, cells) is moved to the bottom of centrifugation tubes. With the development of A-PRF utilizing the low-speed centrifugation concept (LSCC), cell pull-down was reduced, which increased the total number of cells left contained within the top layer of PRF.
The faster and longer centrifugation is carried out, the more cells are pushed to the bottom of centrifugation tubes.
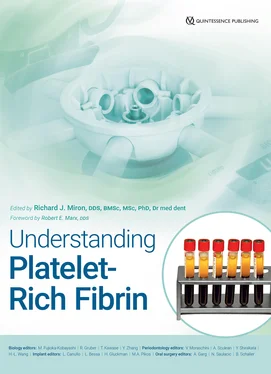
In a cell and GF analysis study on this topic by our group, the newer protocols for the production of A-PRF+, which involves not only lower centrifugation speed but also less time (1300 rpm for 8 min), was shown to lead to even further increases in GF release of TGF-β1, PDGF-AA, PDGF-AB, PDGF-BB, VEGF, IGF, and EGF ( Fig 2-4). 24Therefore, an optimization of centrifugation parameters could be obtained simply by lowering the g-force and time, favoring better GF delivery.
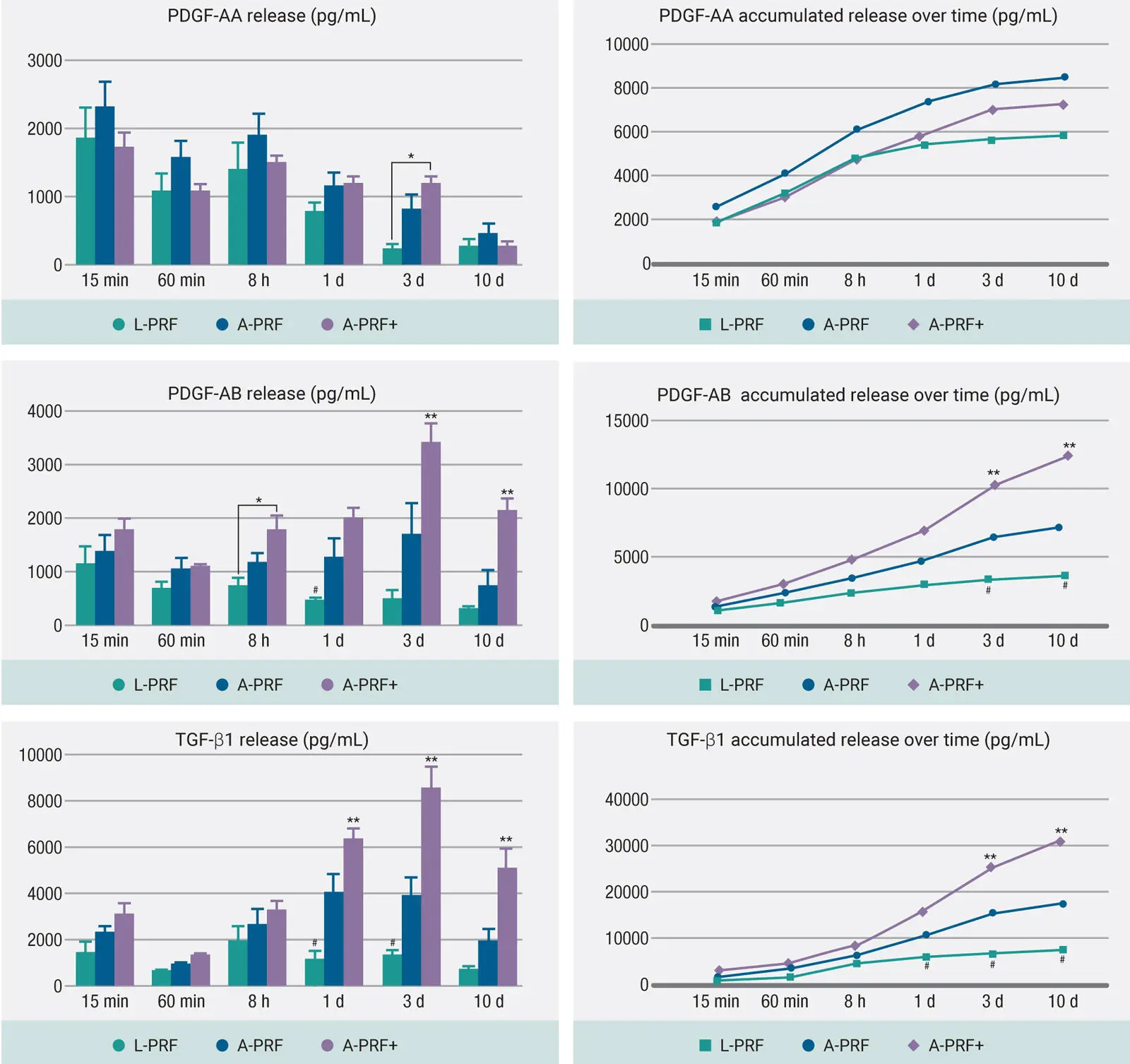
Fig 2-4GF release resulting from the LSCC at each time point for PDGF-AA, PDGF-AB, and TGF-β1 over a 10-day period. In general, it was found that A-PRF+ demonstrated significantly the highest GF release when compared to all other modalities after a 10-day period. (Adapted from Fujioka-Kobayashi et al. 24)
Not surprisingly, when cells were cultured with the various formulations of PRF, it was also observed that centrifugation utilizing these lower speeds/time further led to greater cellular bioactivity. In a study titled “Optimized platelet-rich fibrin with the low-speed concept: Growth factor release, biocompatibility, and cellular response,” our research team demonstrated that protocols at lower speeds and time not only led to significantly greater human fibroblast cell migration and proliferation compared to L-PRF but also demonstrated significantly higher mRNA levels of PDGF, TGF-β, and collagen1 at either 3 or 7 days ( Fig 2-5). In addition, PRF membranes implanted subcutaneously that were fabricated using lower centrifugation speeds also generated greater and faster vascularization in vivo. 24
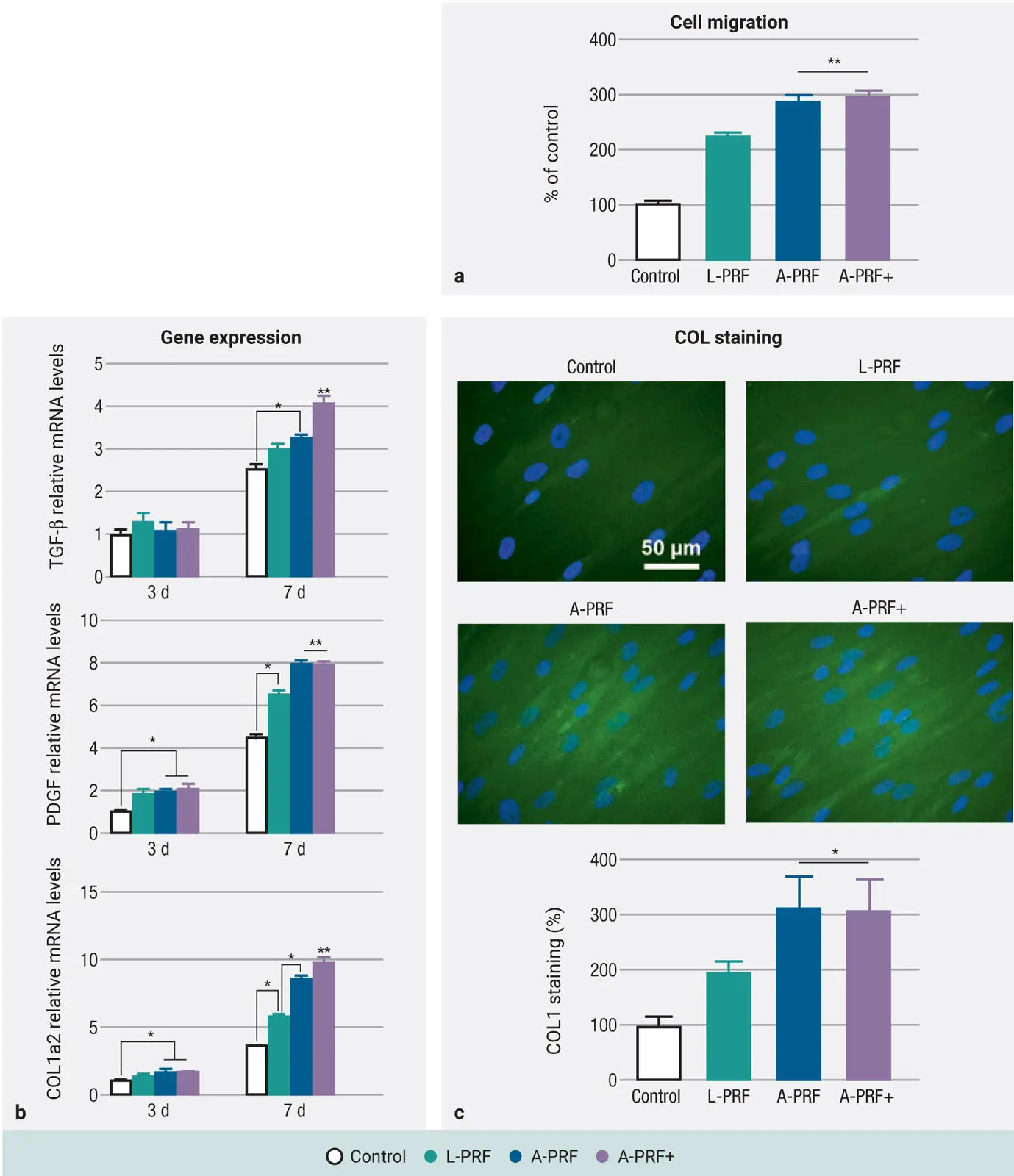
Fig 2-5Human gingival fibroblast behavior exposed to L-PRF, A-PRF, and A-PRF+: (a) Cell migration, (b) gene expression, and (c) collagen synthesis on human gingival fibroblasts. (Adapted with permission from Fujioka-Kobayashi et al. 24)
Comparing Centrifugation Devices
One highly debated topic with much commercial interest has been the role of the centrifugation device on production of PRF. As such, there are general parameters that need to be respected to calculate relative centrifugal forces (RCF) effectively; this forms the basis of an entire chapter in this textbook (see chapter 4). While there are definitely quality differences between centrifugation devices, the debate between which centrifugation system to utilize is generally overhyped, leading to confusion among colleagues regarding how to accurately select, and more importantly, optimize a centrifugation system.
The debate surrounding which centrifugation system to utilize is generally overhyped, leading to confusion among colleagues regarding how to accurately select and, more importantly, optimize a centrifugation system.
As such, in 2018 our research team addressed this question in a study titled “Comparison of platelet-rich fibrin (PRF) produced using 3 commercially available centrifuges at both high (~ 700 g) and low (~ 200 g) relative centrifugation forces” with the specific aim to demonstrate that any centrifugation device could be utilized to produce PRF using the LSCC. 39In that study, PRF was produced on three commonly utilized commercially available centrifuges including the IntraSpin (Intra-Lock), Duo Quattro (Process for PRF), and Salvin (Salvin Dental) devices ( Fig 2-6). Two separate protocols were tested on each machine, including the original L-PRF protocol (~700g RCF-max [~400g RCF-clot] for 12 min) as well as the A-PRF+ protocol (~200g RCF-max [~130g RCF-clot] for 8 min). Each of the tested groups was compared for cell numbers, GF release, scanning electron microscopy (SEM) for morphologic differences, and clot size (both weight and length/width).

Fig 2-6Experimental setup: Each of the centrifuges was utilized in duplicate for a total of six centrifuges. From each patient, a total of 18 tubes were drawn (2 per machine) and centrifuged accordingly at either high- or low-speed protocols. (Reprinted with permission from Miron et al. 39)
It was found that PRF clots produced utilizing the lower centrifugation speeds and time (1) contained a higher concentration of evenly distributed platelets, (2) secreted higher concentrations of GFs over a 10-day period, and (3) were smaller in size. This was irrespective of the centri-fugation device utilized and consistently observed on all three devices. The greatest impact was found between the protocols utilized (up to a 200% difference). Furthermore, it was revealed that centrifugation carried out at lower speeds had a slightly less dense fibrin mesh with more open spaces to allow for cellular migration ( Fig 2-7). Most importantly, it was revealed for the first time that centrifugation tubes actually had a much greater impact on the final size outcome of PRF clots when compared to centrifugation devices. This was the first time our team had observed such dramatic differences in the size outcomes of PRF tubes, and since then a plethora of research has since been performed on that topic alone (see chapter 5).
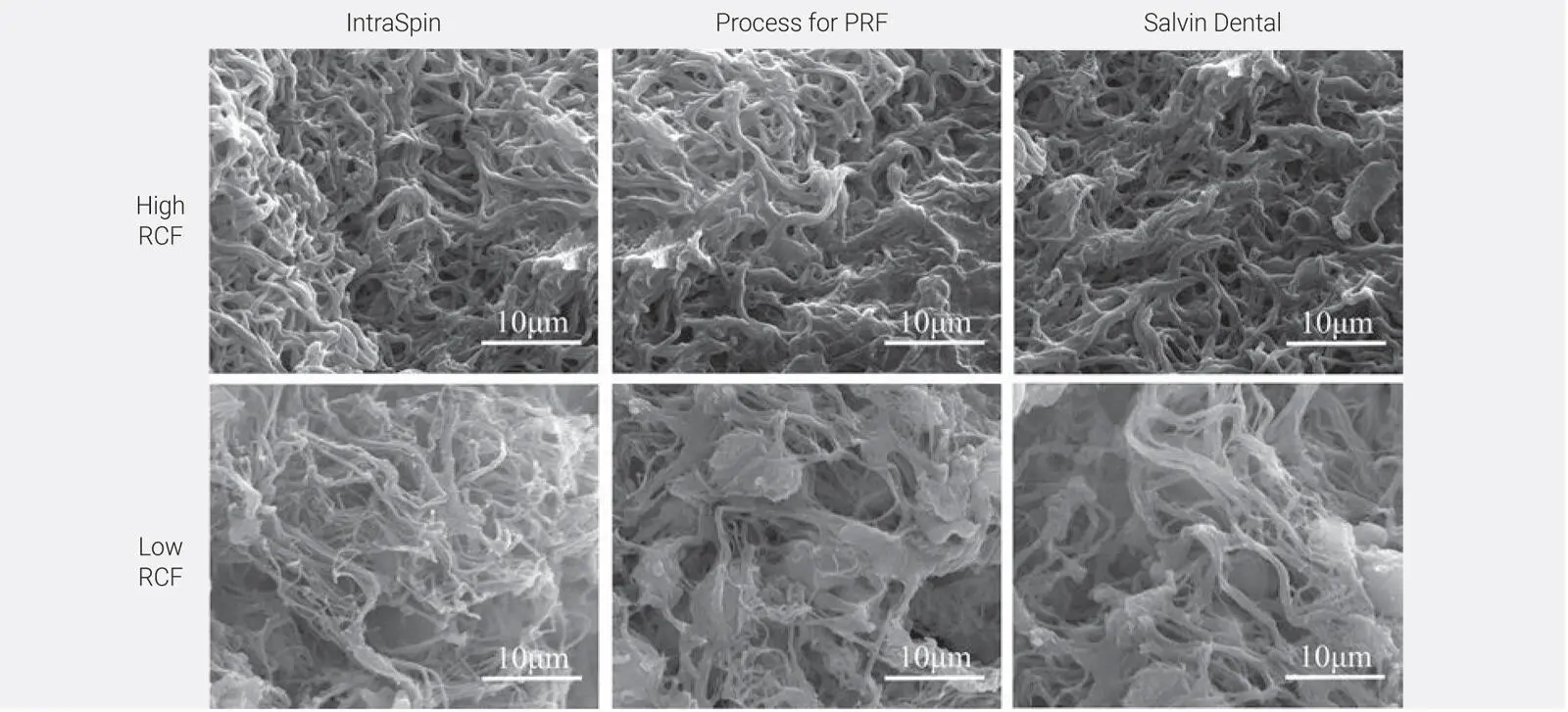
Fig 2-7SEM of PRF clots produced on three different devices at either high-speed (~700g) or low-speed (~200g) protocols. Notice that the clots produced at high g-force typically were more densely packed with fibrin. (Reprinted with permission from Miron et al. 39)
Research has clearly shown that PRF tubes matter much more than the centrifugation device used.
Development of i-PRF
One of the advantages of PRP is that it is liquid in nature, making it easy to utilize in combination with various bone biomaterials, most notably bone grafting materials. With PRF, as centrifugation speeds and times were being reduced further and further, a nonclotted liquid plasma layer was noticed prior to actual clot formation. This liquid-PRF layer is actually liquid fibrinogen that has not yet converted to fibrin. This liquid formulation of PRF was given the working name liquid-PRF or injectable-PRF (i-PRF) for simplicity ( Fig 2-8a). 40This layer can be quickly harvested ( Fig 2-8b) and injected into a defect area. Interestingly, once liquid-PRF has converted to a solid state, it forms the standard fibrin fibrillar PRF that most are familiar with, as depicted in Fig 2-9by SEM. 41Based on its potential for clinical applications, both preclinical and clinical studies have since been conducted to evaluate its regenerative potential.
Читать дальше