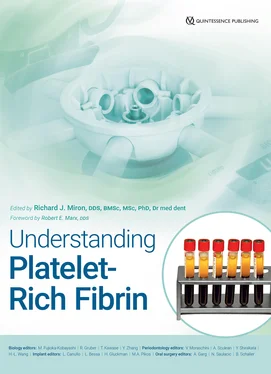
Fig 2-8 (a) Clinical photograph of liquid-PRF. Note that this protocol separates out a small upper liquid-PRF layer about 1 mL in quantity. (b) This liquid i-PRF layer may be harvested into a syringe and utilized as an injectable platelet-rich formulation. (Reprinted with permission from Davies and Miron. 40)
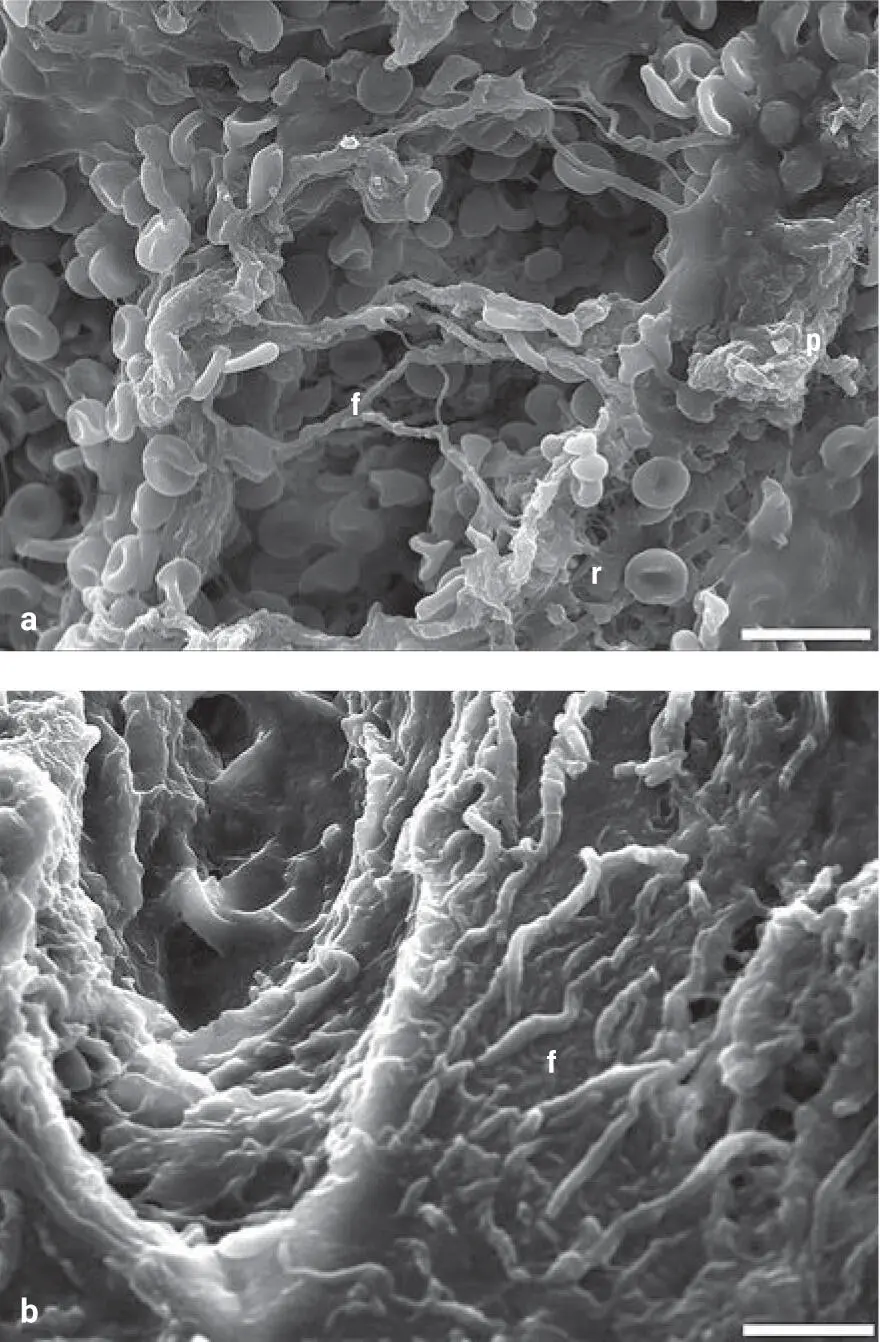
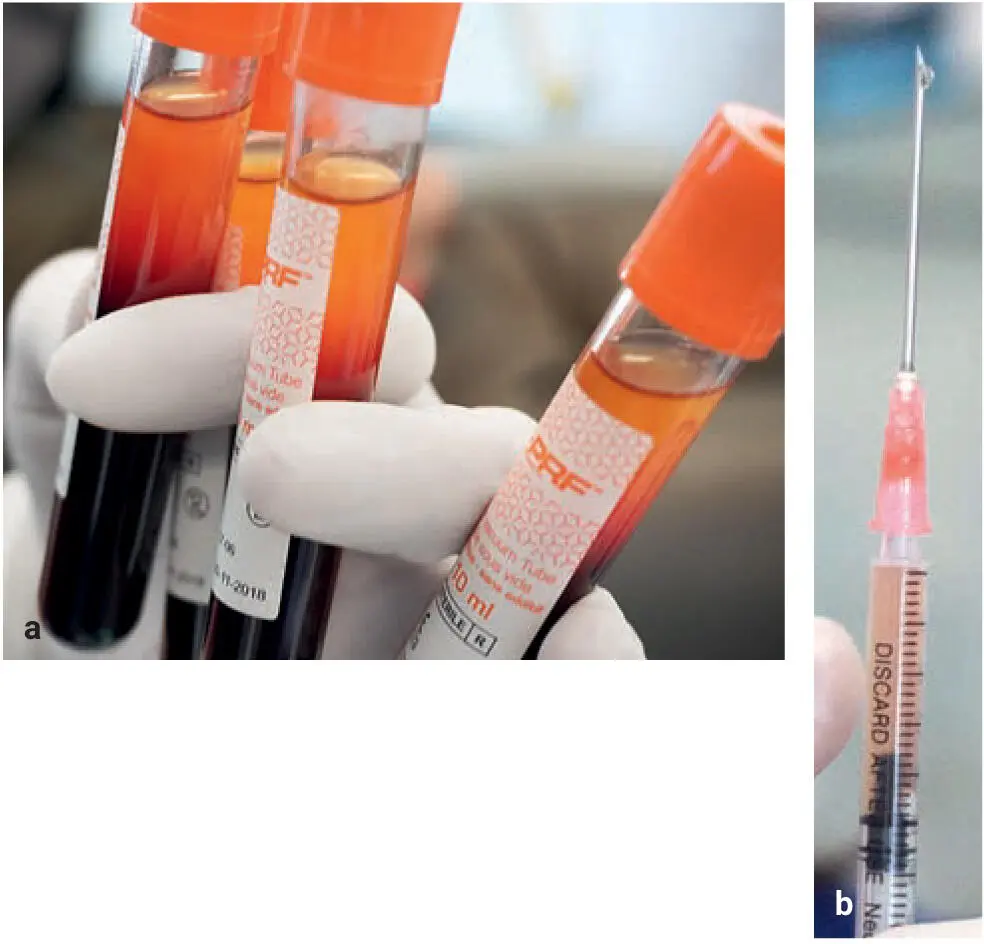
Fig 2-9The surface (a) and cross-section (b) microstructures of the i-PRF. f, fibrin; p, platelet aggregates; r, RBC. Scale bar = 10 μm. (Reprinted with permission from Zhang et al. 41)
Data from our laboratories first found that GF release from PRP was typically within the first hour, whereas i-PRF had a much more widespread release of GFs over time, similar to solid-PRF 42–44( Fig 2-10). Unlike our previous studies comparing various solid-PRF membrane formulations, however, some GFs were in fact secreted in higher levels from PRP when compared to i-PRF, whereas others were more highly released from i-PRF. In 2015, i-PRF was developed and initially studied as a very short and slow centrifugation protocol of 700 rpm (60g) for 3 to 4 minutes. Following this protocol, i-PRF remained liquid for roughly 15 to 20 minutes. This new formulation was utilized for a variety of procedures including mixing with bone grafts to form a stable fibrin graft with improved handling and graft stability.
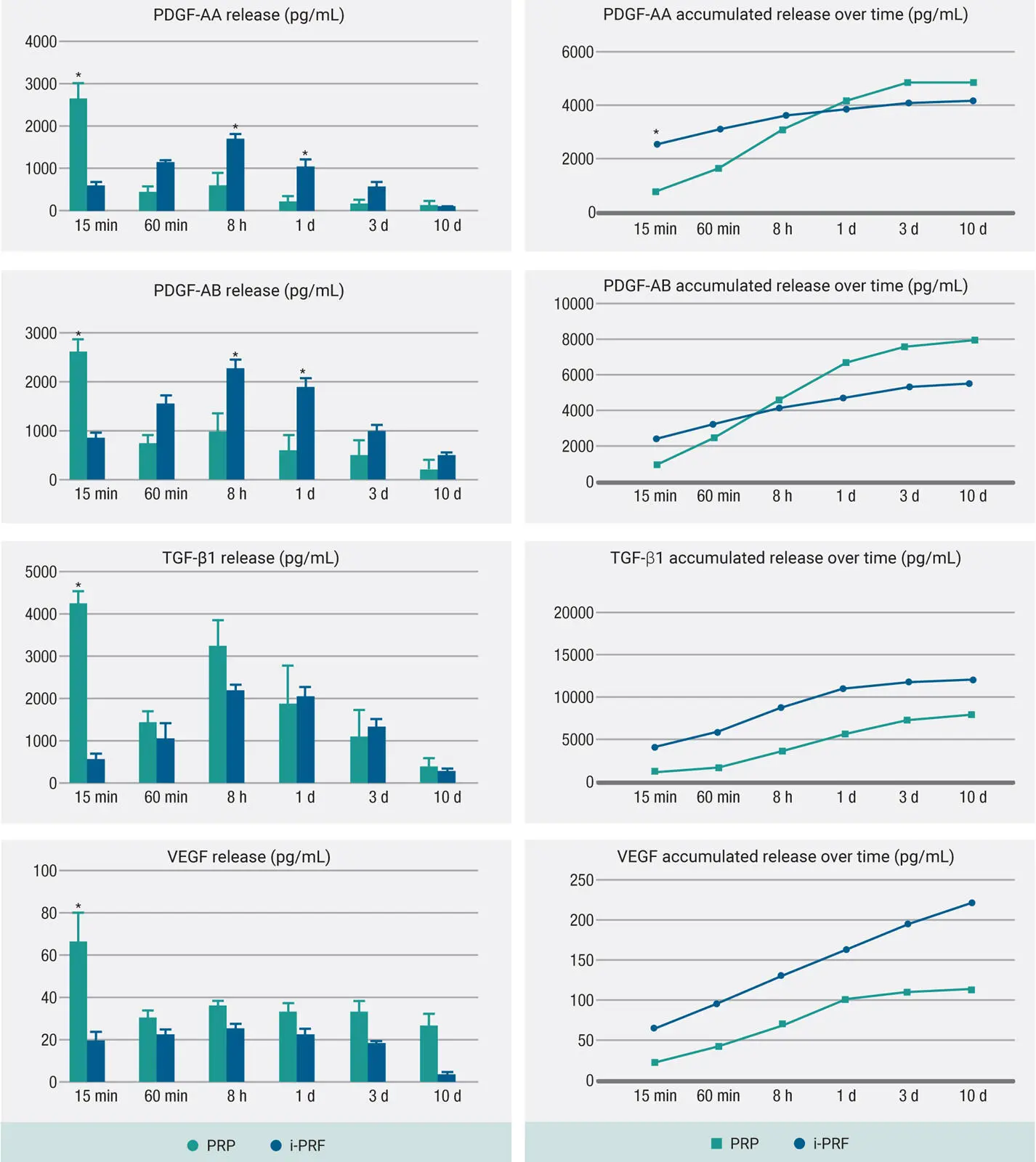
Fig 2-10GF release from i-PRF compared with PRP at each time point for PDGF-AA, PDGF-AB, TGF-β1, and VEGF over a 10-day period. Note the varying GF release from PRP and i-PRF. Some GFs were in fact more highly released from PRP, which posed many questions several years ago. (Adapted from Miron et al. 44)
A variety of basic research studies have since demonstrated the regenerative potential of i-PRF when compared to PRP. 44–49While both formulations exhibited high biocompatibility of human gingival fibroblasts as well as significantly induced higher cell migration when compared to control tissue-culture plastic in vitro ( Fig 2-11), 44it was found that i-PRF induced significantly greater cell migration, mRNA levels of TGF-β, and collagen1 expression. 45–49It wasn’t until years later that further means to improve i-PRF were developed, as highlighted later in this chapter.
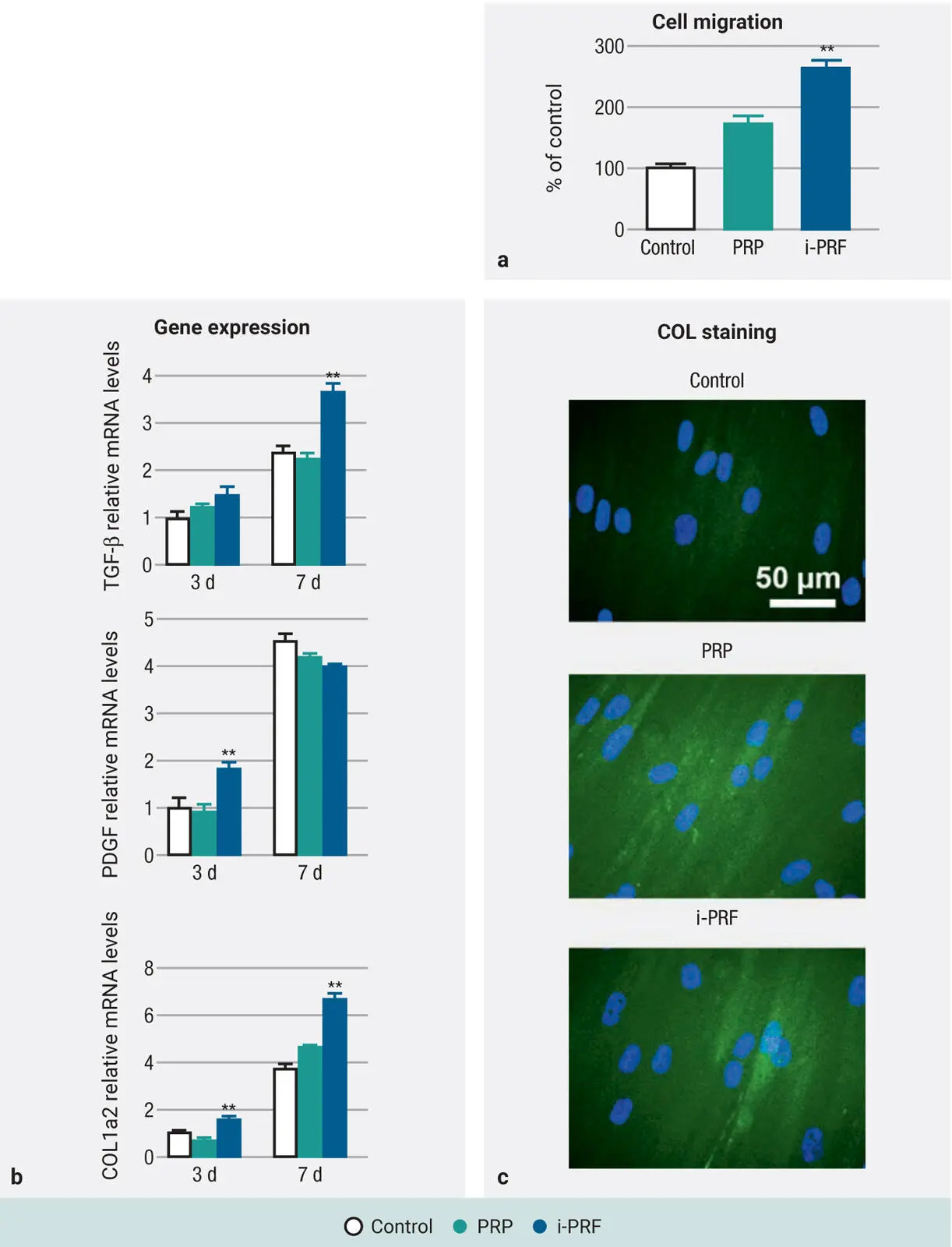
Fig 2-11Human gingival fibroblast behavior exposed to i-PRF versus PRP: (a) Cell migration, (b) gene expression, and (c) collagen synthesis. (Adapted with permission from Miron et al. 44)
Quantifying Cell Types in PRF
Pitfalls of current methods
Two pitfalls exist with quantifying cell types found within PRF research. Histologic studies have been infrequently performed and show variability and bias in results, because many times it becomes difficult to know the exact orientation of PRF relative to the histologic processing. It therefore becomes very difficult to assess where cells are actually located following cell separation and histologic preparation. Another method commonly performed has been the use of complete blood counts (CBCs). Simply, following centrifugation, the plasma layer can be harvested and sent for a CBC and compared relative to whole blood ( Fig 2-12a). While this accurately reports an increased ratio with respect to platelet and leukocyte numbers, one of the main drawbacks is that the method cannot determine if the cells are evenly distributed within the according PRF layers. As illustrated in Fig 2-12b, by utilizing this method, very similar final outcomes were found with only marginal improvements of roughly 20% visible between the two previously utilized protocols of L-PRF and A-PRF, yet histologically a much better distribution of platelets was observed by Ghanaati et al. 6Therefore, a new method was proposed to better quantify cell types in platelet concentrates, as described next.
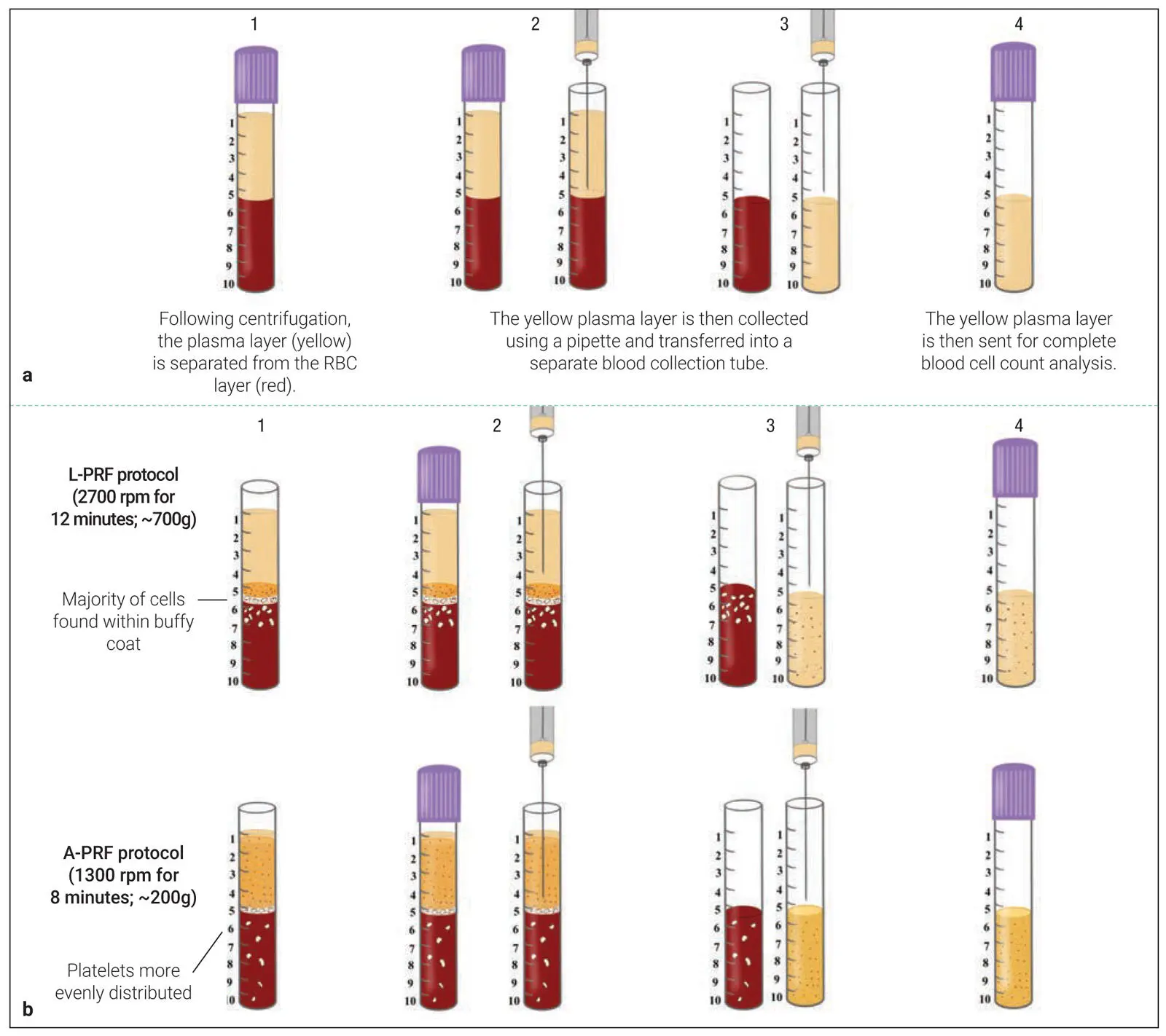
Fig 2-12 (a) One method to investigate cells found in PRP/PRF is to send the plasma layer following centrifugation for a full CBC. This CBC value can be compared to a control of whole blood to investigate its % increase. (b) One of the reported pitfalls of this technique is that the precise location of cells within the upper layer is not revealed. For instance, following the L-PRF protocol, the cells are gathered more precisely at the buffy coat zone, whereas the A-PRF protocol tends to more evenly distribute platelets. Therefore, this technique may not necessarily represent the most ideal scientific accuracy/data.
A novel method to quantify PRF
Over the past few years, several commercially available centrifuges have further been brought to market. These vary in many factors including protocols, RCF values, tube-rotor angulation, rotor radius size, and tube composition. Each of these plays a role in the final obtained PRF membrane, yet little data is scientifically available displaying cell numbers and content in the various layers following centrifugation.
While much commercial debate exists on this topic, there has been no accurate method to quantify/determine the precise location of cells following centrifugation, with few histologic studies performed investigating cell numbers within the fibrin clots. In a pioneering research article published in 2019, our research team proposed a novel method to quantify cell numbers and concentration within the PRF scaffolds following centrifugation by utilizing a sequential pipetting methodology. 50Standard protocols commonly utilized for the production of PRF were investigated according to their respective manufacturer’s protocols. New to this research article, for the first time following centrifugation, 1-mL layers were sequentially pipetted from the upper layer of blood tubes toward the bottom of the tube until all 10 mL were harvested in sequential samples ( Fig 2-13). 50Each of these 10 samples from each centrifugation tube was then sent for CBC analysis to accurately quantify the cell numbers within each 1-mL blood layer and then compared according to cell numbers and concentrations. This study represented a novel experimental methodology to more accurately depict cell numbers in PRF following centrifugation using various protocols.
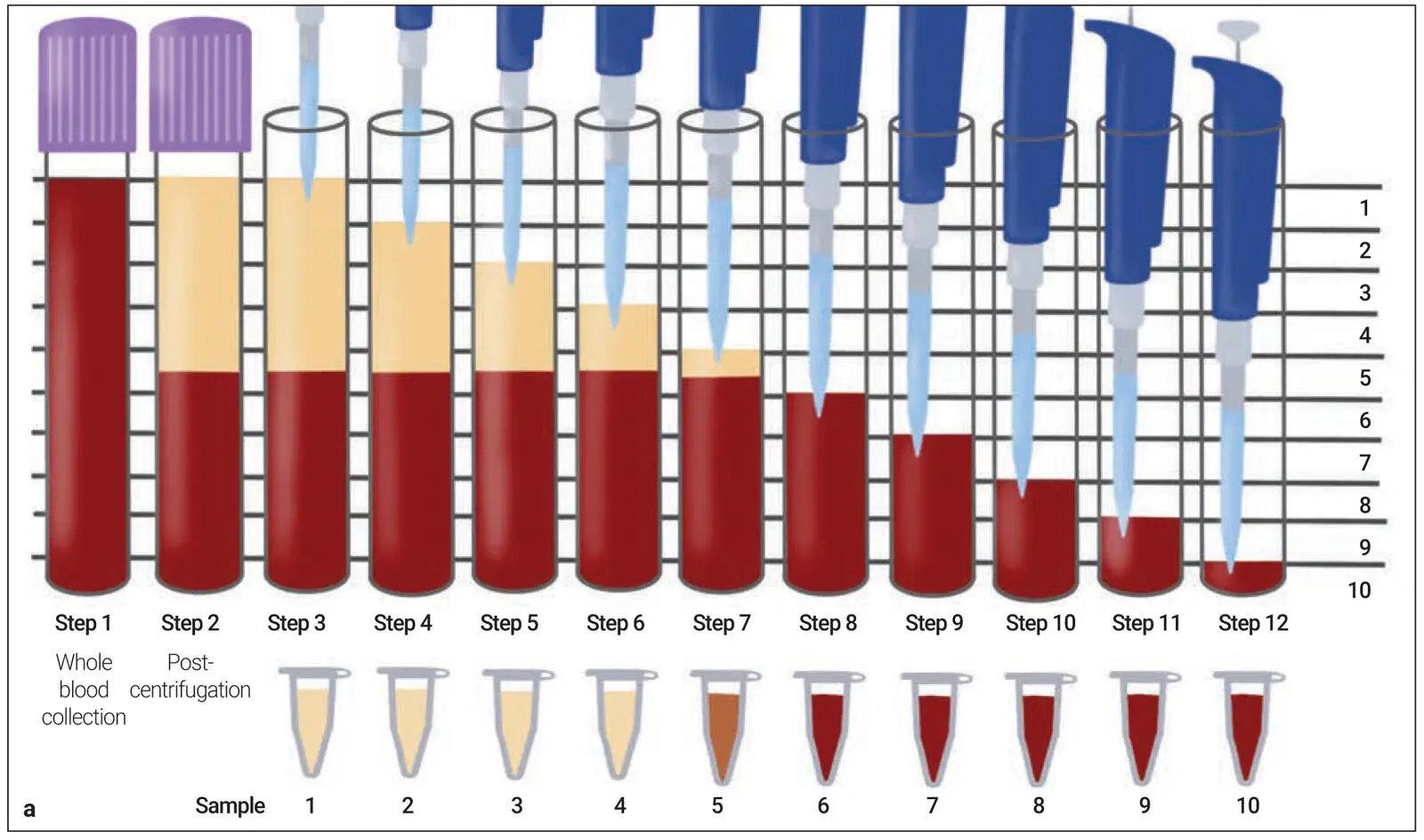
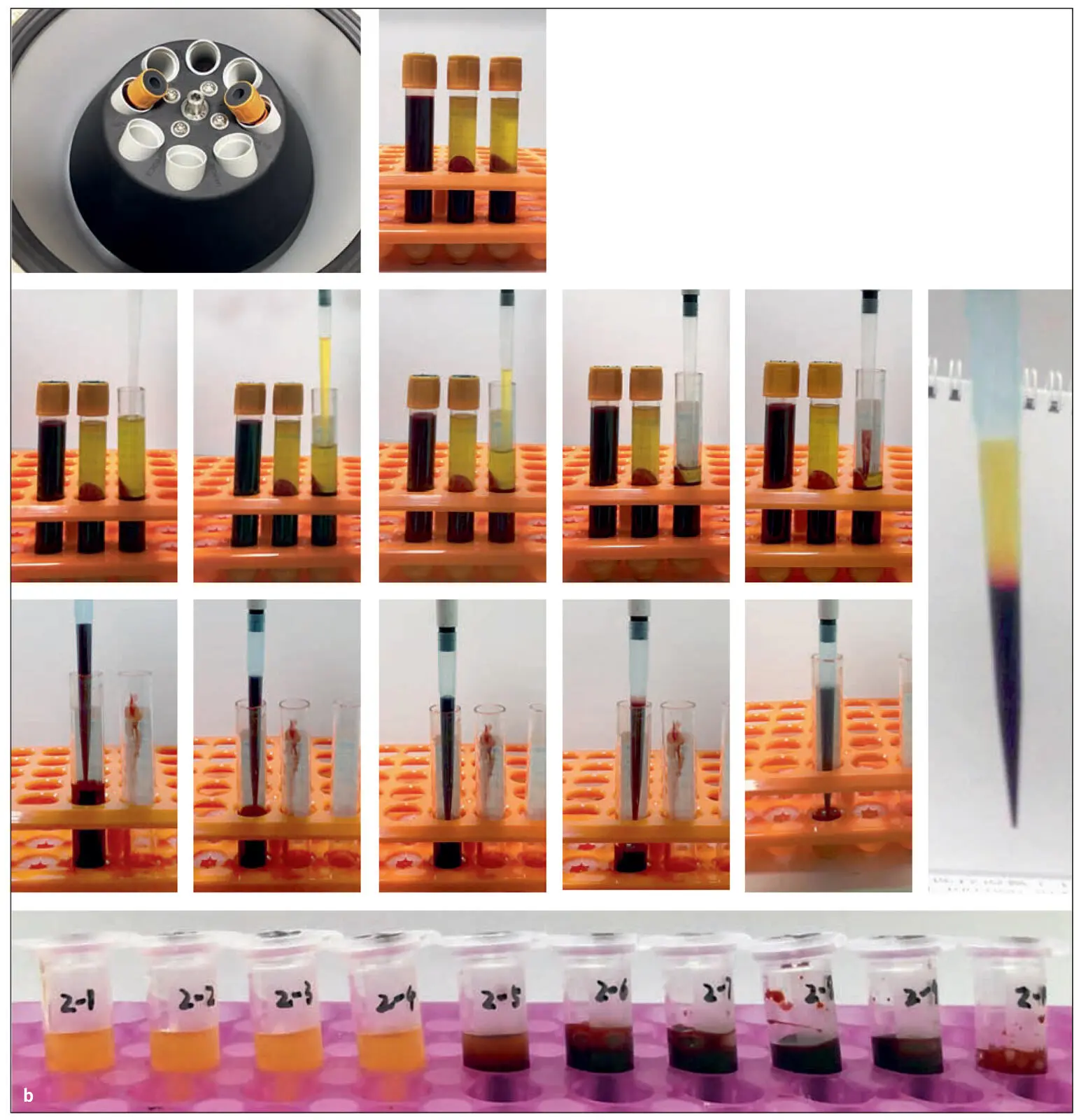
Fig 2-13 (a) Illustration demonstrating the proposed novel method to quantify cell types following centrifugation of PRF. Currently, one of the limitations is that whole blood is compared to the total plasma concentration following centrifugation. This, however, does not give a proper representation regarding the location of cells following centrifugation. By sequentially pipetting 1 mL of volume from the top layer downward, it is possible to send each of the 10 samples for CBC analysis and accurately determine the precise location of each cell type following centrifugation at various protocols. Notice that one layer (in this case layer 5) will contain some yellow plasma and RBCs. This is typically the location of the buffy coat, where a higher concentration of platelets is located. (b) Visual demonstration of the protocol. Following centrifugation with two 10-mL centrifugation tubes, blood layers are then separated. Thereafter, 1-mL samples are pipetted precisely from the upper layer downward. Notice that when layer 5 was drawn, it was possible to visualize the layer separation between the yellow plasma and RBC layers. This separation layer was noted for all samples. (Reprinted with permission from Miron et al. 50)
Читать дальше