Despite its growing success and continued use after its discovery, several reported limitations existed with these initial formulations of PRP. The 30-minute or longer technique was generally considered lengthy for routine dental or medical practice, and more importantly, the use of anticoagulants was shown to limit wound healing from reaching its maximum potential. Simply put, when injury is created following an open wound, a blood clot is one of the first steps that occurs in order for healing to take place. Shortly thereafter, cells and GFs get trapped within this newly formed ECM, and the wound healing process/cascade begins. By limiting the body’s ability to form a stable clot, wound healing is limited. Several studies have now demonstrated the superior outcomes of platelet-rich fibrin (PRF) when compared to PRP simply by removing anticoagulants from their formulations. 17–21Even the pioneering research team behind the plasma rich in growth factors (PRGF) concept (Anitua et al) have since demonstrated more physiologic healing ability with anticoagulant removal. 17
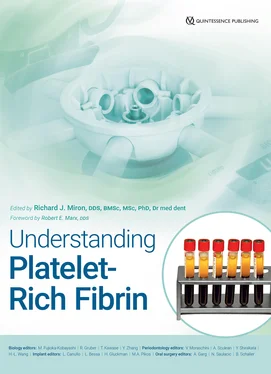
Another drawback of PRP was the fact that it remained liquid by nature (due to the use of anticoagulants), so when it was combined with biomaterials, a much faster delivery of GFs was observed ( Fig 1-3). While an initial burst of GFs is typical of PRP therapy, a slower release of GFs over an extended period of time has been shown to better stimulate cell growth and tissue regeneration. 22,23
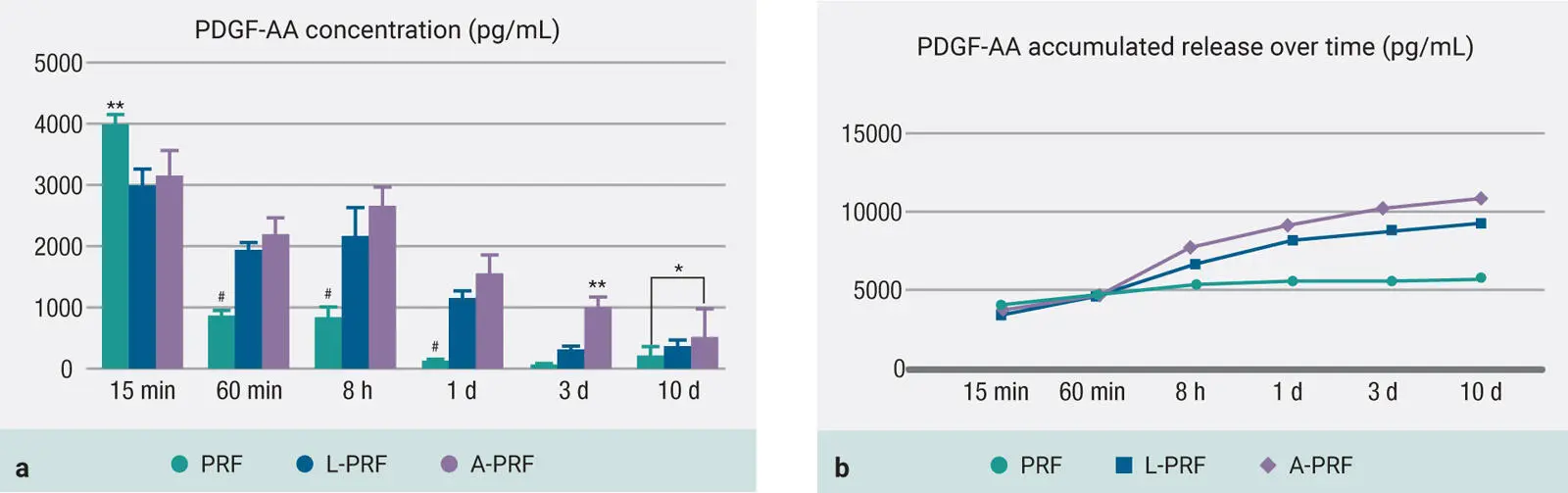
Fig 1-3 (a and b) GF release from PRP and PRF at each time point of PDGF-AA over a 10-day period. Notice that while PRP has significantly higher GF release at early time points, over a 10-day period, significantly higher levels are most commonly found with A-PRF due to the slow and gradual release of GFs utilizing slower centrifugation speeds. (Adapted from Kobayashi et al. 19)
Much advancement related to PRP therapy has been made over the past 20 years, and two excellent textbooks have been written by its pioneers— Dental and Craniofacial Applications of Platelet-Rich Plasma by Robert E. Marx and Arun K. Garg (Quintessence, 2005), and Autologous Blood Concentrates by Arun K. Garg (2018). Its breakthrough features include the novel ability to concentrate platelets to supraphysiologic doses and further stimulate tissue regeneration across virtually all tissue types. For these reasons, PRP has not surprisingly been utilized in practically every field of medicine.
Snapshot of PRP
Marx was the first to show that a concentration of platelets could favor tissue regeneration in the oral cavity.
A subsequent device was brought to market thanks to these breakthrough research projects conducted at the University of Miami (Harvest system).
PRP is credited for having exponentially grown the entire field of platelet concentrates, including its subcategories such as PRF.
L-PRF (2000–2010)
Because the anticoagulants utilized in PRP prevented clotting, pioneering work performed by Dr Joseph Choukroun and Dr David Dohan Ehrenfest led to the development of PRF. 24The aim was to develop a second-generation platelet concentrate focused on anticoagulant removal. Because anticoagulants were removed, a much quicker working time was needed, and centrifugation had to begin shortly after blood draw (otherwise, the blood would naturally clot). Furthermore, high g-force centrifugation protocols were initially utilized in an attempt to separate blood layers prior to clotting. The final spin cycle (initial studies ranged from 2500–3000 rpm for 10–12 minutes = ~700g) resulted in a plasma layer composed of a fibrin clot with entrapment of platelets and leukocytes. The main advantage of this fibrin matrix was its ability to release GFs over an extended period of time while the fibrin clot was being degraded. 25Over the years, PRF has been termed L-PRF (for leukocyte platelet-rich fibrin ) due to the discoveries that several leukocytes remained incorporated in PRF and that white blood cells play a central and key role in the tissue healing process. The most commonly utilized protocol today is a spin cycle at 3000 rpm for 10 minutes or 2700 rpm for 12 minutes (RCF-max = ~700g, RCF-clot = ~400g).
Several other advantages also existed during clinical use because it avoided the need for dual-spin protocols requiring pipetting or various specialized tube compartments, which made the overall procedure much more user-friendly, cheaper, and faster when compared to PRP. Original protocols were purposefully designed to spin at high centrifugation speeds with the main aim of phase separation to occur as quickly as possible in order to separate the red corpuscle base layer from the upper plasma layer prior to clotting. Following centrifugation, a platelet-rich fibrin mesh was formed, giving it the working name PRF 26–28( Fig 1-4). PRF has since been highly researched, with over 1,000 publications dedicated to this topic alone.
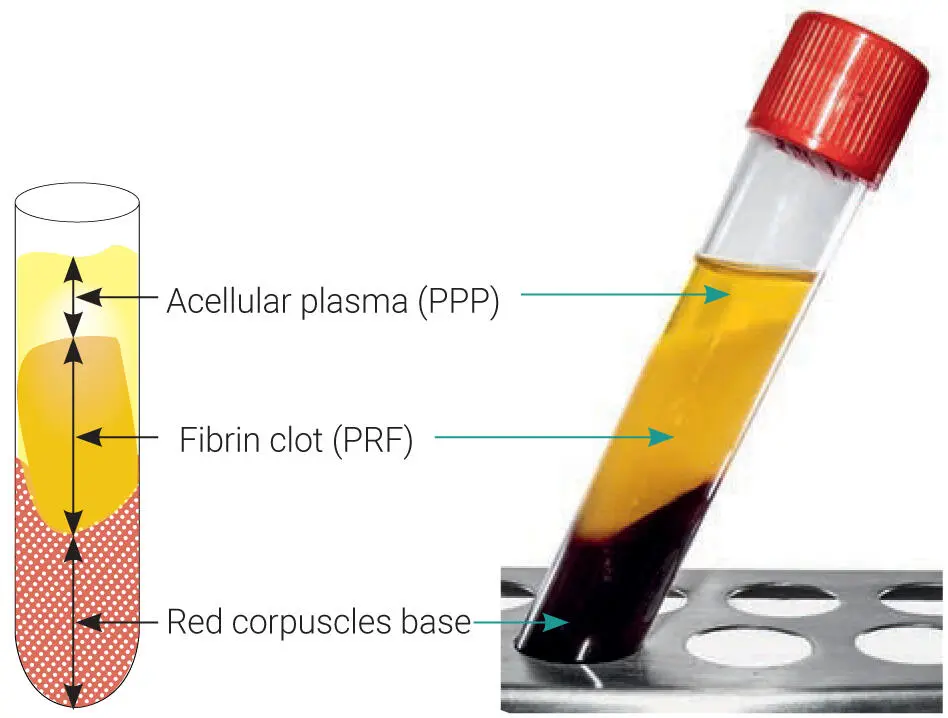
Fig 1-4Layers produced after centrifugation of whole blood. A PRF clot forms in the upper portion of tubes after centrifugation.
Additionally, research teams from around the world have demonstrated the impact of leukocytes on tissue healing. 29–34While it was once thought that the additional benefit of leukocyte incorporation into PRF was its main properties in improved host defense to foreign pathogens, 29–34it has since been shown in well-conducted basic research studies that leukocytes are pivotal to tissue regeneration and favor faster wound healing also. 11,35–37In dentistry, where the oral cavity is filled with bacteria and microbes, the inclusion of leukocytes was initially thought to play a pivotal role in wound healing by participating in the phagocytosis of debris, microbes, and necrotic tissues, as well as directing the future regeneration of these tissues through the release of several cytokines and GFs and orchestrating cell-to-cell communication between many cell types.
Tissue engineering with PRF
Tissue engineering has been an emerging discipline over the past decade, with major breakthroughs routinely being made every year. At its simplest foundation, tissue engineering requires three parameters: (1) a scaffold responsible to support tissue ingrowth, (2) cells that may act to promote tissue regeneration, and (3) GFs that stimulate the overall wound healing events. Unlike the majority of biomaterials currently available on the market, PRF actually contains each of these three properties ( Fig 1-5). For comparative purposes, routine bone allografts contain a scaffold (mineralized cortical/cancellous bone) and GFs embedded in its bone matrix (such as bone morphogenetic protein 2 [BMP-2]) but have no cells. Recombinant human GFs typically have a GF (for instance, rhBMP-2) and a carrier (collagen sponge) but also lack cells. Certain stem technologies typically contain cells and also a delivery system (for instance a nanocarrier delivery system) but lack GFs. The ability to actually contain each of the three tissue engineering properties within a single biomaterial is quite rare and, more importantly, usually extremely expensive (think recombinant GFs and/or stem cell technology).
Fig 1-5Three main components of PRF all derived naturally from the human body. These include (1) cell types (platelets, leukocytes, and red blood cells); (2) a provisional ECM 3D scaffold fabricated from autologous fibrin (including fibronectin and vitronectin); and (3) a wide array of over 100 bioactive molecules, including most notably PDGF, TGF-β, VEGF, IGF, and EGF.
Читать дальше