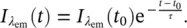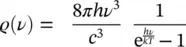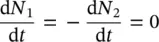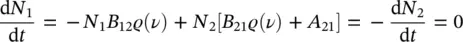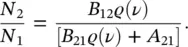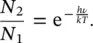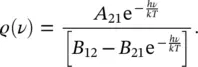In considering the phenomenology of the emission process, we can distinguish two kinds of phenomena. Both of them are related to the emission of light, but their time dependence is very different. In particular, one emission process lasts for a long time (more than μs) after the removal of exciting light (like in the luminescence emergency panels) and is called phosphorescence. The other rapidly (less than 0.1 μs) decreases in amplitude as the exciting light is turned off and is known as fluorescence. An experimental procedure to distinguish these processes consists in recording the emission amplitude at a given wavelength as a function of time after the excitation light is turned off. In Figure 1.3is reported the result of a typical time‐resolved photoluminescence experiment. The excitation light impinges on the sample up to the time 1 ms, to let the system reach a stationary state, and the emission is recorded at a selected wavelength λ em. The emission intensity amplitude recorded is constant, in accordance to the stationary state. At the time t 0= 1 ms, the excitation is rapidly removed by turning it off or by an opportune shutting system. The signal is then continuously recorded at t > 1 ms at λ emand it is found that its amplitude decreases. In the figure is reported a typical decay with single exponential law:
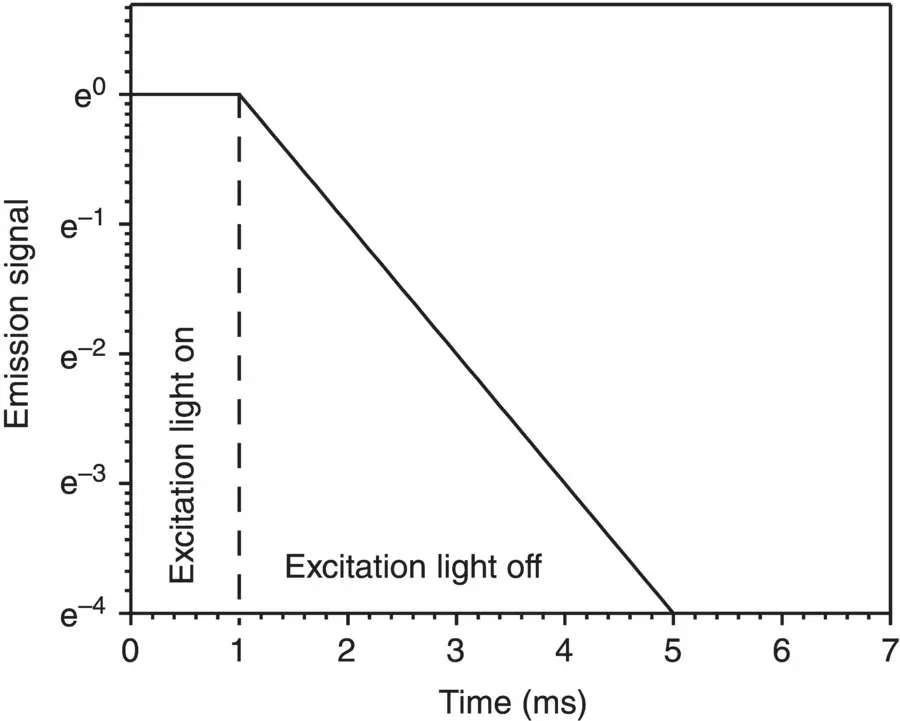
Figure 1.3Typical decay curve as a function of time of the emission. For t < 1 ms, the exciting light is illuminating the sample, for t ≥ 1 ms it is turned off.
(1.23) 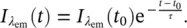
By this procedure, it is possible to record the characteristic time τ that defines the lifetime, or the decay time, of the photoluminescence, and obtain the time needed to reach a value of the emission amplitude 1/e of its stationary value in a given experiment [2, 10]. Values of τ up to ~10 ns are typical of fluorescence phenomena and larger lifetimes, up to 10 3s, are characteristic of phosphorescence, enabling empirically to distinguish them [2, 5, 10]. In the following, a microscopic interpretation of the reported phenomena is given. It is worth mentioning that specific instrumentations are needed to carry out time‐resolved photoluminescence [2, 5].
1.2 Microscopic Point of View
The empirical observations of absorption and emission phenomena contain very important information on the electronic and molecular properties of matter. In this view, it is fundamental to understand what kind of knowledge can be obtained from such experiments. In this paragraph, the theoretical bases that enable to determine microscopic features about the electronic states from the macroscopic measurements will be deepened.
1.2.1 Einstein Coefficients
A simplified model of the atom constituted by two nondegenerate energy levels is assumed to evaluate the interaction with radiation. As reported in Figure 1.4, the lower energy level is E 1and the higher energy level is E 2. This system interacts with the thermal equilibrium radiation field at temperature T .
According to Planck’s theory, the energy distribution of the radiation is given by [8, 11, 12]
(1.24) 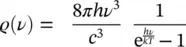
where ρ ( ν ) is the density of energy for unit volume and unit frequency interval, h the Planck’s constant, ν the radiation frequency, c the speed of light, k the Boltzmann’s constant (1.38 ⋅ 10 −23J K −1), and T the absolute temperature.
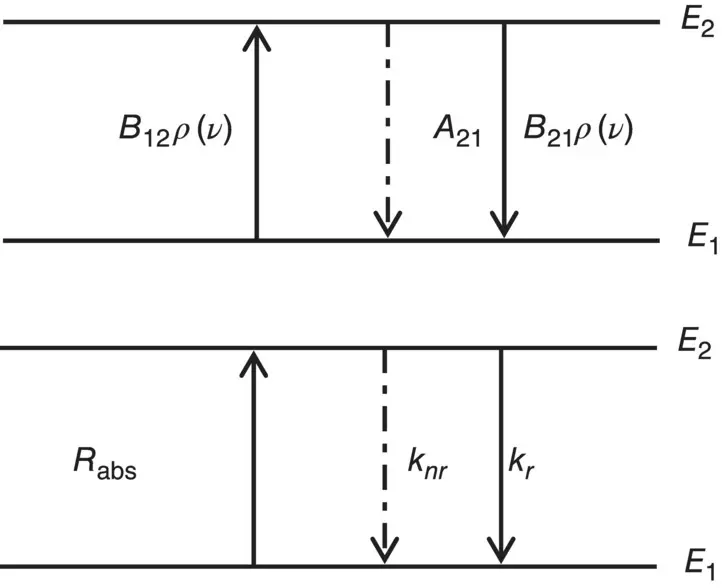
Figure 1.4Top: Schematic representation of two energy‐level atom. The arrows represent the absorption and emission transition processes whose probability is given by Einstein’s coefficients A 21, B 12, B 21and the density of radiation ρ ( ν ). Bottom: Schematic representation of two energy levels of an atom. The arrows represent the excitation and relaxation transitions. The absorption rate is R abs, the radiative emission rate is k r, and the non‐radiative transition rate is k nr.
The interaction between a density N of atoms for unit of volume and the radiation field causes an exchange of energy with those electromagnetic waves’ modes having frequency related to the atom’s energy levels’ separation by the equation E 2− E 1= hν . As a consequence, based on Einstein’s treatment, the following processes can occur [8, 13]:
Transition from the state E 1 to the state E 2, stimulated by the absorption of a photon; based on Einstein’s theory, the rate of this process is given by(1.25)
where N 1is the density of atoms (population) in the lower energy state.
Transition from the state E 2 to the state E 1, stimulated by the emission of a photon; the rate is given by(1.26)
where N 2is the density of atoms in the upper energy state.
Spontaneous transition from the state E 2 to the state E 1 with a rate(1.27)
The Einstein’s coefficients A 21, B 12, and B 21have been used. In particular, it is worth observing that B 12and B 21are related to the presence of the field (stimulated processes of absorption and emission, respectively), whereas A 21is present also without electromagnetic field and is related to spontaneous emission. This term is related to the radiative emission lifetime introduced in the previous paragraph and, in detail, it is the reciprocal of the lifetime at low temperature, A 21= 1/ τ [13]. At thermal equilibrium, the population of atomic states should reach a stationary condition and it is expected that
(1.28) 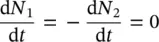
and, based on the above reported processes, one obtains
(1.29) 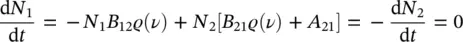
and the relation
(1.30) 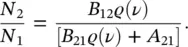
The Boltzmann distribution at thermal equilibrium in a two‐level system without degeneracy predicts that [14]
(1.31) 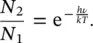
Equating (1.30)and (1.31)and solving with respect to ρ ( ν ), it is found that
(1.32) 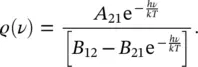
Читать дальше