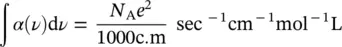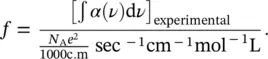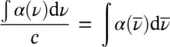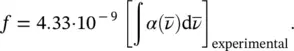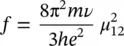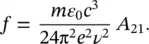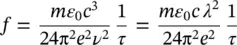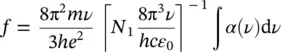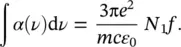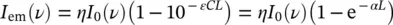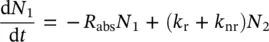1 ...7 8 9 11 12 13 ...46
1.2.2 Oscillator Strength, Lifetime, Quantum Yield
In the previous paragraph, we have determined theoretical quantities relating the atomic wavefunctions of the energy levels to spectroscopic observables. In the simplified model of the atom with a single electron, it is considered that this latter can oscillate in a harmonic potential well. The atomic system is then a charged harmonic oscillator. The wavefunctions of this system enable to evaluate the electric dipole matrix element μ 12and to determine the theoretical integrated absorption reported by (1.52). It can be shown that the expected value of integrated absorption is [5, 11]:
(1.53) 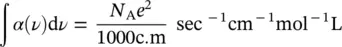
where N Ais Avogadro’s number, c is the speed of light, and m is the electron mass (9.109 ⋅ 10 −31kg). Equation (1.53)gives a numerical value that can be compared with experimental results. This comparison gives origin to the quantity called oscillator strength and usually given by f :
(1.54) 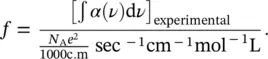
The oscillator strength is a dimensionless quantity characterizing the transition between the two considered energy levels E 1and E 2. By introducing [5, 11]
(1.55) 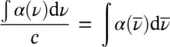
it is shown that
(1.56) 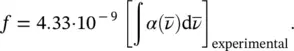
Expected values of f are lower equal than unity and on decreasing of the probability of the absorption process decreases too. This feature is linked to the selection rules that highlight those quantum transitions between energy levels giving an electric dipole matrix element different from zero [5].
Another form of the oscillator strength is [8, 15]
(1.57) 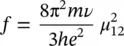
which, on the basis of (1.34), (1.35)and (1.44), can be written
(1.58) 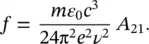
Considering that A 21= 1/ τ [13], as stated above, it is shown that
(1.59) 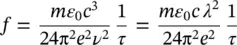
connecting the oscillator strength to the radiative emission lifetime at low temperature. Furthermore, using (1.52)to determine μ 12, Eq. (1.57)becomes
(1.60) 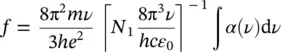
giving
(1.61) 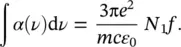
This formula relates the integrated absorption coefficient to the concentration of absorbing centers through the oscillator strength. In particular, given a concentration N 1,the area of the absorption curve is higher at larger oscillator strength. Furthermore, both the oscillator strength and the concentration of absorbing centers can be experimentally determined from this formula once one of the two parameters is known. This result shows the relevance of the absorption measurements and once more the exploitability of this experimental technique to determine microscopic information about the matter.
In general, the relation between absorption and emission processes can be described by
(1.62) 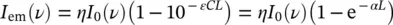
where the absorption effect is reported using (1.5)and (1.8)and differences in absorption and emission frequency ν have been neglected, assuming that one is the inverse process of the other. The above formula shows that the number of emitted photons by a sample of thickness L depends on the number of photons impinging on the sample, I 0, and the number of them giving absorption effect, as evidenced by the terms in parenthesis. The efficiency of emission is determined by η, which is called (external) radiative quantum efficiency or quantum yield [2, 10, 16]. It is worth noting that in the case of low absorption effect, εCL ≪ 1, α L ≪ 1, the emission is proportional to the concentration of absorbing species. The quantum efficiency of photoluminescence is defined as the ratio of the number of emitted photons to the number of absorbed photons
(1.63) 
In the interaction process between radiation and a two‐level atom, in which the absorption drives the electron from the starting state E 1to the final state E 2at higher energy, as reported in Figure 1.4, not all of the absorbed photons give rise to an emitted photon since other processes could drive the excited electron back to the E 1state. Based on the above considerations, it is clear that η ≤ 1. To go deeper into the connection between η and other physical parameters, it is useful to consider the simplified energy level scheme of the two‐level atom reported in Figure 1.4. The absorption process, in which the electron is promoted to the E 2state, is represented by the vertical arrow connecting E 1and E 2states. This process has a rate, probability per unit of time, R abs. The system in the excited state is out of thermal equilibrium because typically E 2− E 1> kT . As a consequence, the electron returns to the lower energy level. This relaxation process could occur by the emission of a photon with frequency ν = ( E 2− E 1)/ h , denoted as radiative process with a rate k r. Furthermore, the electron could relax without emission of photons, not radiatively, by exchanging energy with its environment with a rate k nr. The rate equation for the variation of the population N 1of the state with energy E 1can be written as
(1.64) 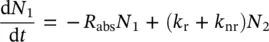
where the population of the excited state N 2has been introduced. This equation is a balance between absorbed photons and relaxation from the excited state through radiative (emitted photons) and non‐radiative processes. Under stationary equilibrium conditions, there will be no change in the populations with time and it is found that
Читать дальше