The TPE ammonium 13was tested for the detection of fish sperm DNA. It was found that cis ‐ 13had a high sensitivity (139 pM) and high‐intensity ratio I max/ I 0(6.6), whereas a mixture of cis / trans ‐ 13about 1 : 1 displayed a low‐sensitivity (326 pM) and low‐intensity ratio I max/ I 0(4.5), indicating that the cis ‐isomer was a much better DNA sensor than the trans ‐one due to more restriction of intramolecular motion including the double bond rotation (see Figure 3.23).
There are more examples in which TPE cycle at the cis ‐position could emit fluorescence in solution due to the restriction of double bond rotation. Rathore et al. synthesized a class of stilbenoprismands 14via an intramolecular McMurry coupling reaction, which contained a TPE core‐bearing cycle at the cis ‐side (see Figure 3.24left) [46]. While the parent TPE showed no detectable emission, the solution of 14showed significant emission under similar conditions although there were two substituted phenyl rings that could freely rotate. By the direct coordination of TPE tetraimidazolium salts with silver or gold, Hahn and Strassert [47] prepared TPE dicycles 15on the cis ‐side. The TPE dicycles 15showed strong emission in solution but TPE tetraimidazolium salts emit no fluorescence, demonstrating the important role of the RDBR process on the fluorescence (see Figure 3.24right).
Hu et al. [48] reported one TPE derivative dicycle 17, in which two pairs of the phenyl rings at the cis ‐position were connected by two diacetylenes. While the no cyclized TPE reactant 16emitted very weak fluorescence in solution, the emission of 17is very strong even in dilute solution. This fluorescence enhancement after cyclization at the cis ‐position should mainly come from the RDBR process (see Figure 3.25).
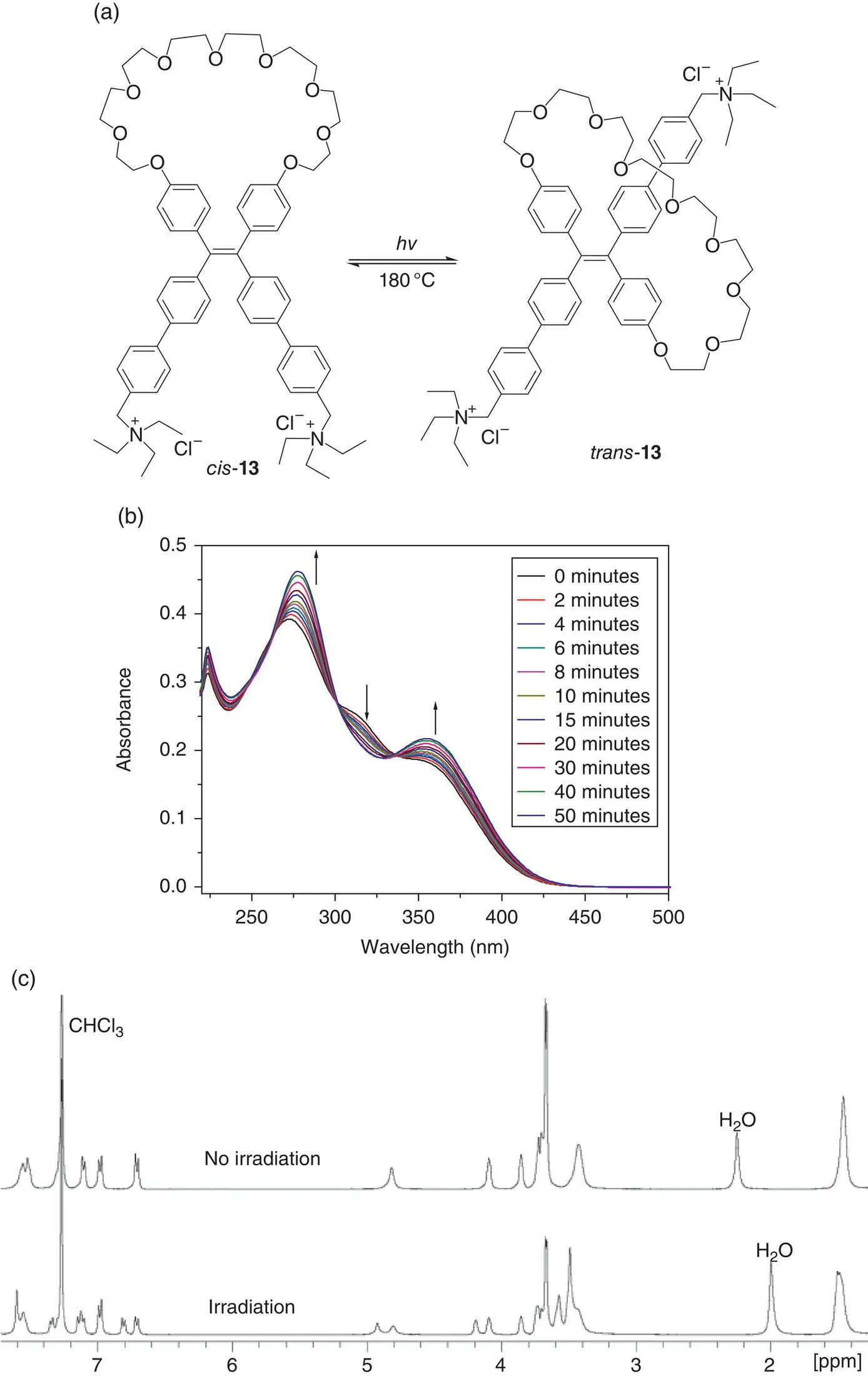
Figure 3.23 (a) The cis‐ / trans ‐isomerization of 13under a 365‐nm light irradiation and heating. (b) Change in UV–vis spectrum of compounds cis‐ 13in dichloromethane under an irradiation of 365‐nm light from fluorophotometer for different periods. [ cis ‐ 13] = 1.0 × 10 −5M. (c) The 1H NMR spectra of cis ‐ 13in CDCl 3before and after irradiation by a 365‐nm portable UV lamp for one hour.
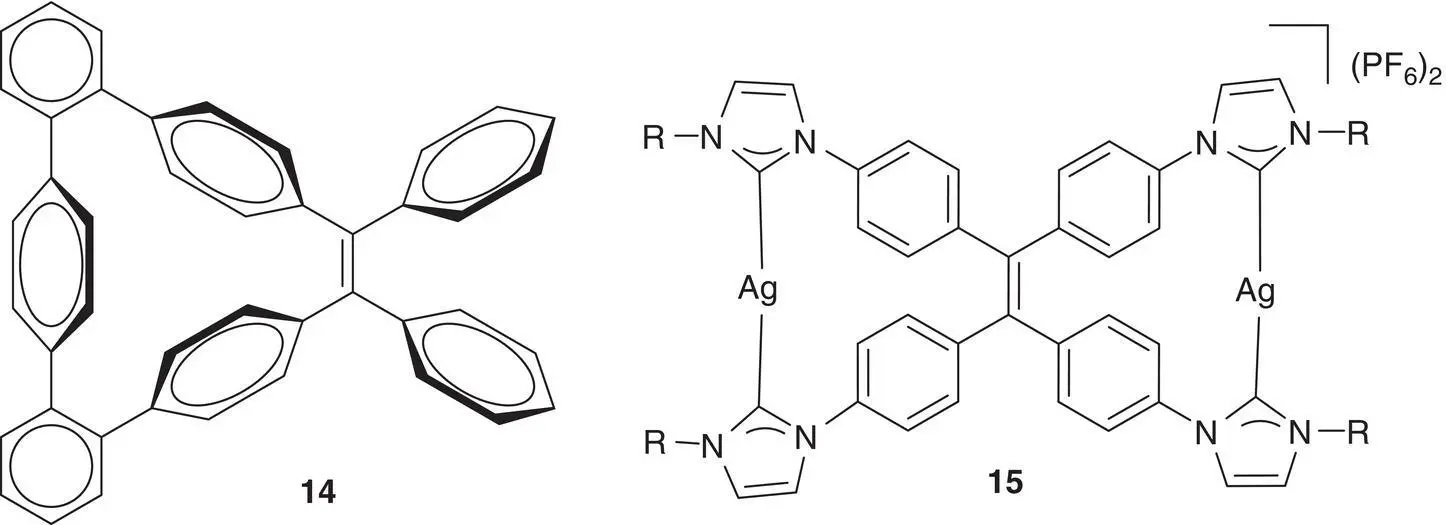
Figure 3.24 The structure of AIEgens 14and 15.
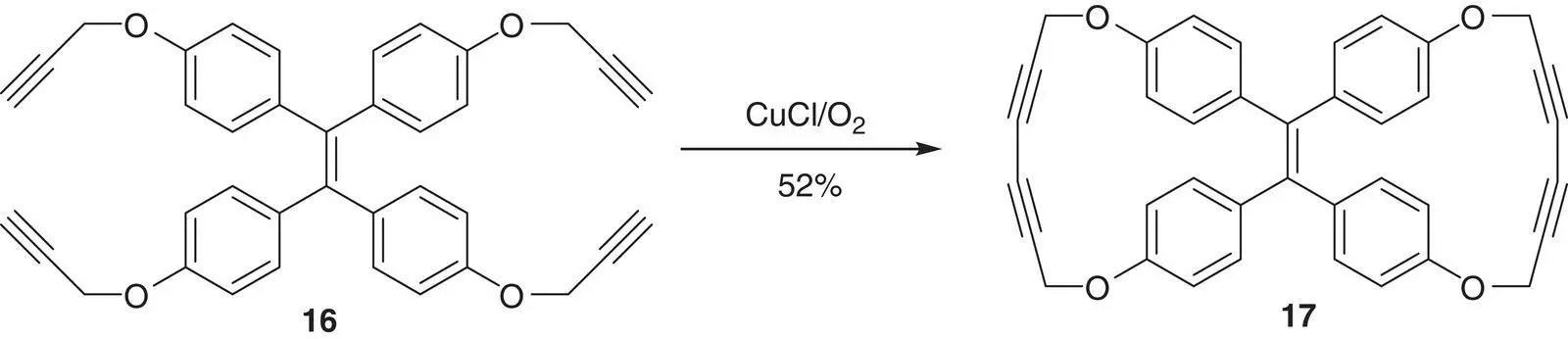
Figure 3.25 The synthesis of the emissive molecule 17.
3.2.4 Research of Theoretical Calculation on RDBR
The effect of restriction of the double bond rotation can be shown intuitively from the experimental phenomenon, but to understand in more details the behavior of the double bond in the excited state and the role it plays, more theoretical studies are needed. In this regard, quantum‐computational simulation and ultrafast time‐resolved spectroscopy are two major methods. The former can simulate the changes in molecular energy and structure during the fluorescence process, by comparing the energy barriers of different decay routes in the excited state to find where the nonradiative relaxation takes place. The latter can probe and resolve the excited‐state dynamics and reaction processes by monitoring the structural changes and the emergence of new species, finding nonradiative process [49, 50].
Through computational studies, detailed activities of the molecule in the excited state can be described, especially in single‐molecular behaviors, which is similar to the state of AIEgens in solution. In this part, an emerging theory called the restricted access to conical intersection (RACI) mechanism has been used for explaining the AIE effect of many AIEgens. Some conical intersections (CIs) are blamed for the weak emission of AIEgens in solution, such as π twist, photocyclization, ring puckering, excited‐state intramolecular proton transfer (ESIPT), bond stretch, and so on [51–53]. Recent computational studies have contributed to the clarification of the excited‐state decay process of TPE in the solution state, and the results indicated the presence of a CI in the ultrafast quenching process of TPE including photocyclization and/or π twist, rather than the propeller‐like rotation of the side phenyl groups. Despite an ongoing debate, there are reports revealing that the twist of the double bond is a typical CI for the deactivation of TPE. Excitation to the S 1state (HOMO → LUMO) causes an elongation of the double bond, which initiates the twisting dynamics. This motion stabilizes the first excited state (S 1) and destabilizes the ground state (S 0), ultimately causing the two states to become degenerate, which are referred to as CI (see Figure 3.26). The dynamic process explains subsequent E – Z photoisomerization and weak emission of isolated TPE molecules.
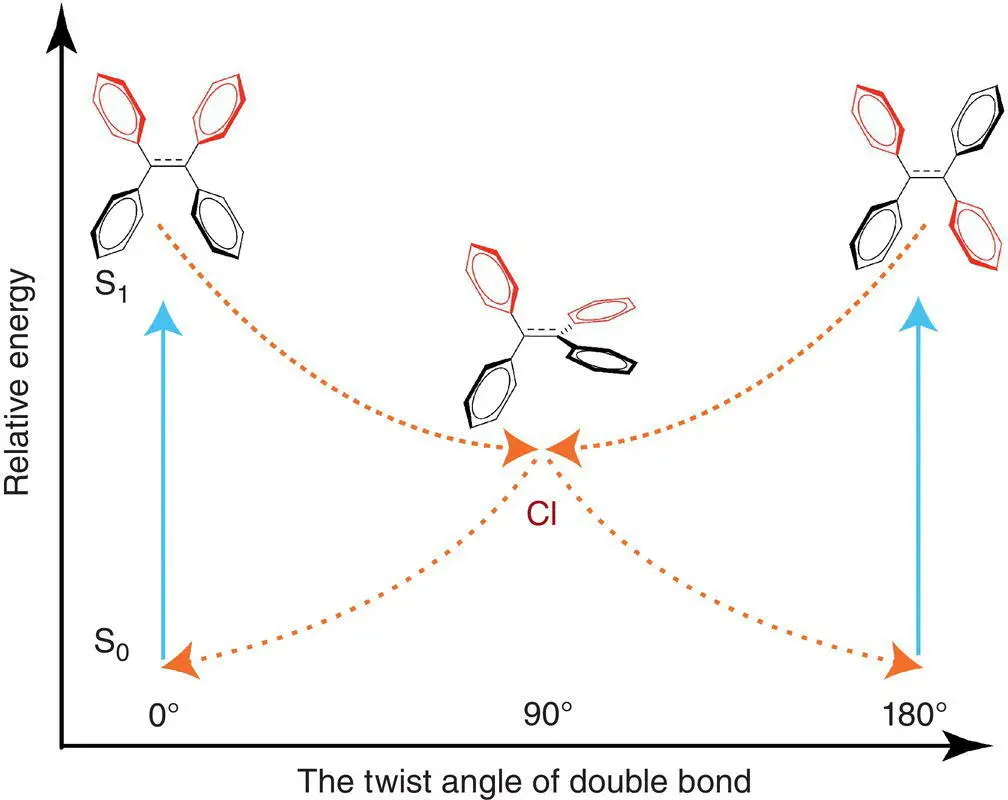
Figure 3.26 A brief illustration of the conical intersection (CI) process through the rotation of double bond in the excited state for TPE in solution.
Zhao et al. [54] report results of the semiclassical simulation study of the excited‐state dynamics of photoisomerization of TPE. By monitoring the change of the length with time, the stretching vibrational mode of ethylenic bond in the excited state was examined. When TPE was excited by a femtosecond laser pulse, the central double bond was excited to stretch from the initial 1.37 to around 2.20 Å in 300 fs. Then, the twisting motion of the fully extended double bond was activated by the energy released from the relaxation of the stretching mode, until the central double bond formed a perpendicular formation and gave an ethylenic bond twisted about 90°. This process was completed in 600 fs, and this twisted structure remains approximately until about 4800 fs. At 4800 fs, a nonadiabatic transition to the electronic ground state occurred. The results of the simulation clearly showed that the ethylenic bond twisting takes place in the subpicosecond scale. This research first revealed the important influence of twisting of the ethylenic bond on the nonradiative decay of the photoexcited TPE at molecular levels through the employment of computational studies.
Corminboeuf et al. [55] descripted excited‐state dynamics of isolated TPE through trajectory surface hopping (TSH) simulations using linear response time‐dependent density functional theory (TD‐DFT) within the Tamm–Dancoff approximation (TDA) at the PBE0/def2‐SVP level. By analyzing motion trajectories, they found that the excited TPE undergoes an ultrafast CI to the ground state through the rotation of the excited double bond. As shown in Figure 3.27, with the rotation of the C═C bond, the energy of the initial excited state (S 1, red curve) continued to decrease, but the ground‐state (S 0, magenta curve) energy was increasing. After ~1 ps, the S 1state became nearly degenerate with the ground state and eventually reached the CI between the S 1and S 0states. To efficiently reach the S 0/S 1, CIs were responsible for fluorescence quenching after TPE photoexcitation in solution. However, there were more trajectories (75%) that decayed to the ground state through photocyclization. The author also found that the two processes are incompatible. The phenyl rings were initially close to one another and cyclization dominated. As the twisting motion around the central double bond proceeded, the cyclization became inaccessible and another decay channel (ethylenic twist) opened.
Читать дальше
















