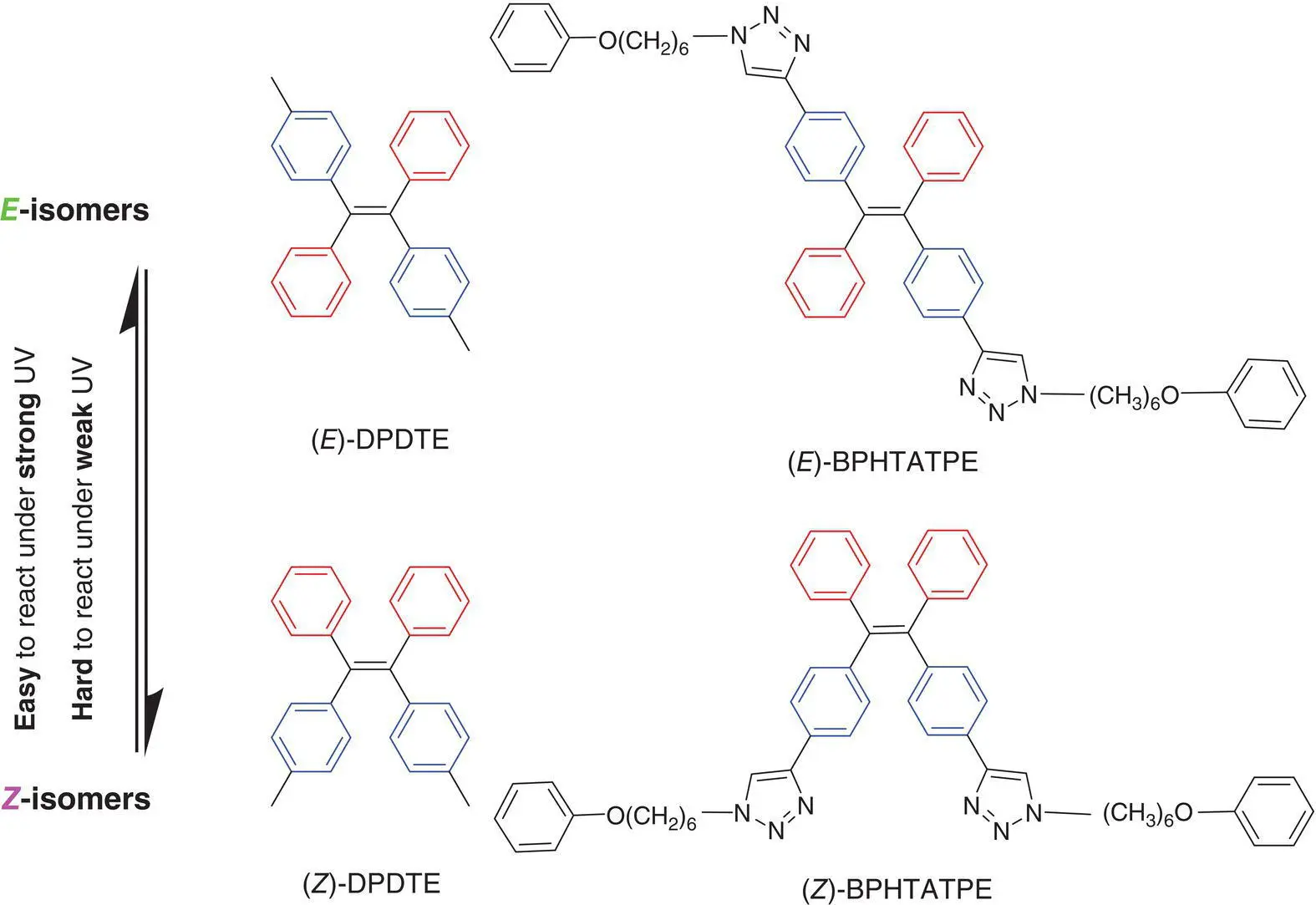
Figure 3.13 The molecular structures of DPDTE and BPHTATPE.
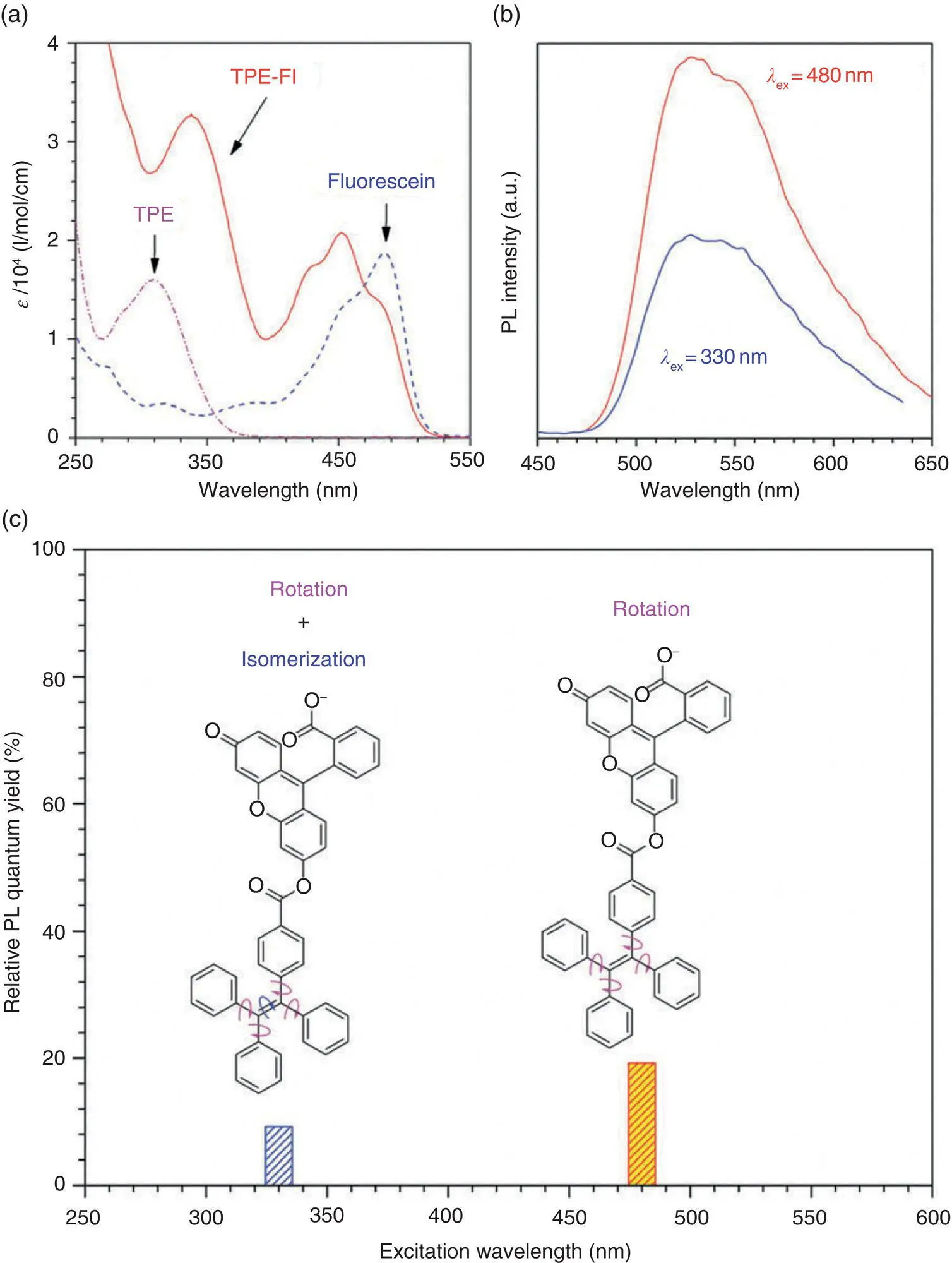
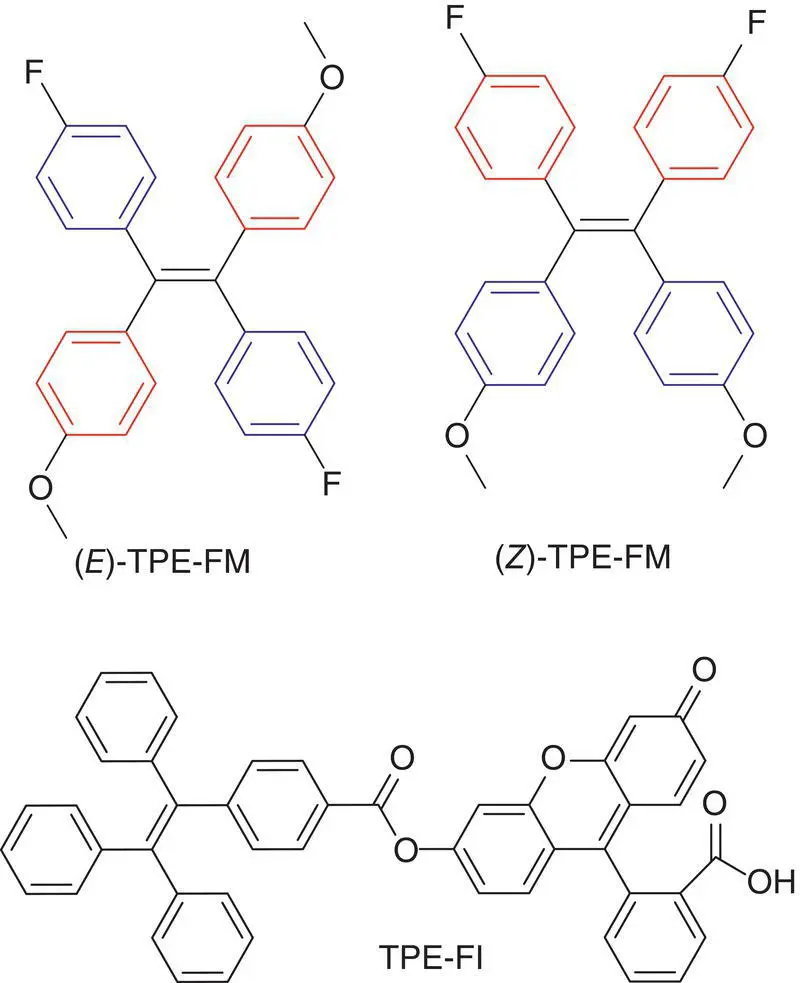
Figure 3.14 Structure of TPE‐FM and TPE‐Fl. (a) Absorption spectra of TPE, TPE‐Fl, and fluorescein. (b) Emission spectra of TPE‐Fl in solution ( λ ex: excitation wavelength). (c) Relative fluorescence quantum yields of TPE‐Fl.
Source: Reproduced with permission from Ref. [42]. Copyright 2016, Royal Society of Chemistry.
In order to disclose the different roles of the double bond rotation and phenyl ring rotation on the AIE process, Tang et al. elaborately designed a TPEgen‐bearing fluorescein unit (TPE‐Fl). This TPEgen displays two main absorption bands at 340 and 450 nm, which are from TPE and fluorescein units, respectively (see Figure 3.15). In solution, TPE emits a weak fluorescence at 490 nm, which is overlapped with the main absorption band of the fluorescein unit. Therefore, the emission wavelength of the TPE unit can act as the excitation wavelength of the fluorescein unit through a fluorescence resonance energy transfer process. Therefore, by using a two‐color excitation, the contribution of the double bond rotation and phenyl ring rotation can be determined. When TPE‐Fl was excited by a long wavelength of 480 nm, the decrease of the fluorescence intensity of TPE‐Fl compared to that of the fluorescein only came from the phenyl ring rotation because the double bond rotation would not occur under so long wavelength of light. When TPE‐Fl was excited by a short wavelength of 330 nm, the decrease of the fluorescence intensity of TPE‐Fl compared to that of the fluorescein came not only from the phenyl ring rotation but also from the double bond rotation because the double bond would break under a high energy of light. By taking the fluorescence quantum yield ( Φ f) of fluorescein to be 100%, the Φ fwith excitation wavelengths of 480 and 330 nm were 19.2 and 9.2%, respectively. It suggested that the fluorescence was decreased by 10% due to double bond rotation but the fluorescence decrease due to the phenyl ring rotation was 100–19.2% = 80.8%. From this indirect experimental evidence, they concluded that the double bond rotation played a minor role on the AIE effect.
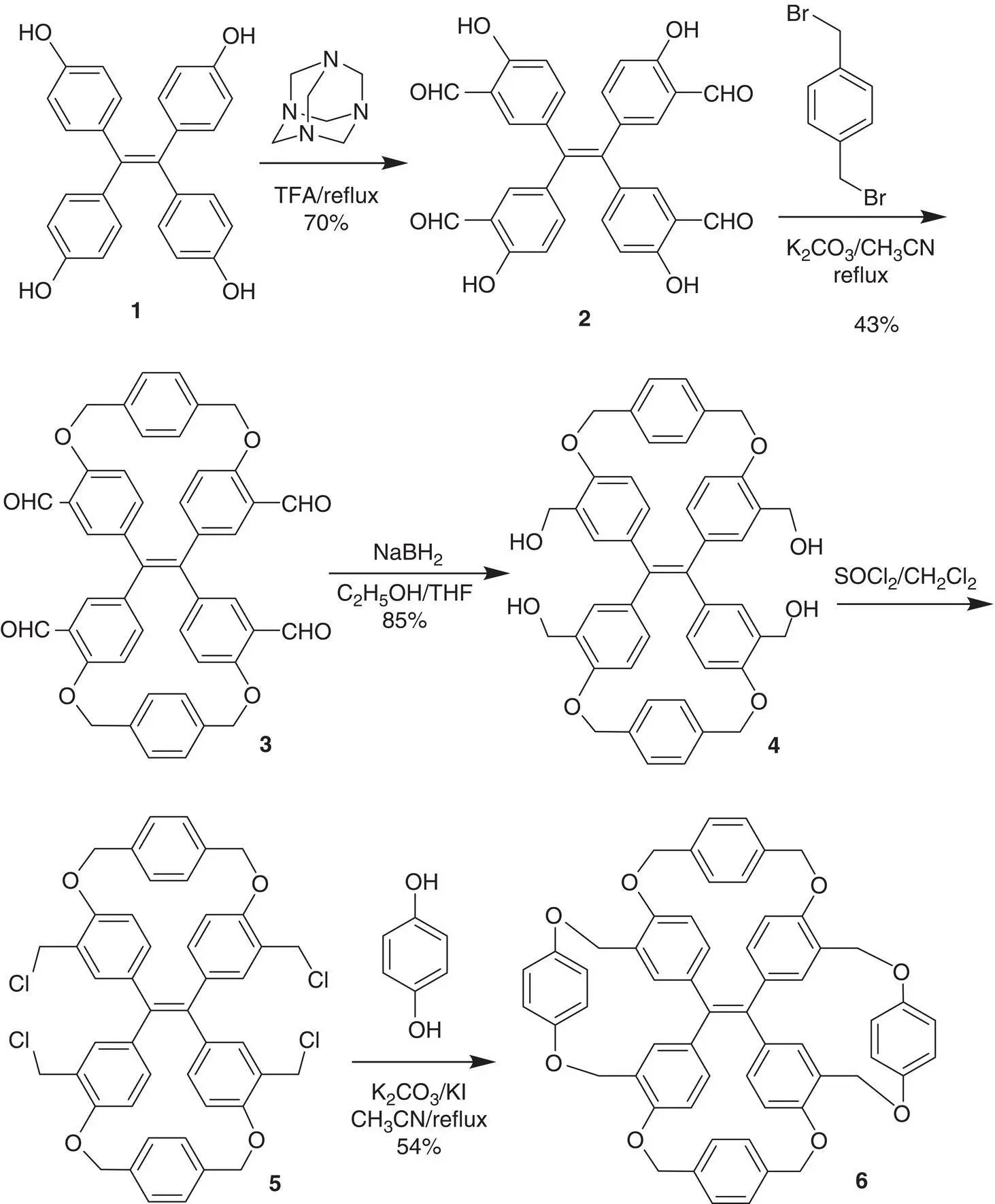
Figure 3.15 The synthetic route of cis ‐TPE dicycles 3– 5and TPE tetracycle 6.
Indeed, in the ground state, phenyl rotation is much easier than the C═C bond, but during the fluorescent course, the double bond can be excited and break to generate radical or charged species (radical, cation, and/or anion), its twist or torsion become available, which make the true radiation‐less relaxation pathway become quite complicated.
3.2.3 Investigating of RDBR AIE Mechanism by Immobilization of TPE Propeller‐like Conformation
In addition to the observation of the EZI process that can disclose RDBR mechanism, immobilization of TPE propeller‐like conformation, especially cyclization of TPE at cis ‐position, can be used to explore the RDBR process. After bridging between two phenyl rings of TPE at the cis ‐position, the double bond will be unable to freely rotate due to the restriction of the bridge chain. But the phenyl rings can still freely rotate. Therefore, the effect of RDBR on the fluorescence will be clearly observed.
In 2016, Zheng’s group [43] found an ideal route for the synthesis of cis ‐TPE dicycle in which the double bond rotation can be blocked at the excited state (see Figure 3.15). By intramolecular nucleophilic substitution of 2with 1,4‐bis(bromomethyl)benzene, cis ‐TPE dicycle tetraldehyde 3could be obtained in a 43% yield. The formation of cis ‐TPE dicycles between two phenyl groups at the cis ‐position instead of those at the gem ‐position was ascribed to the length of 1,4‐benzenedimethyl tether that was more compatible with the distance between two cis ‐phenyl rings than that between two gem ‐phenyl ones. With this key intermediate in hand, even TPE tetracycle 6whose propeller‐like conformation was completely immobilized was obtained.
Noticeably, while the TPE derivatives 1and 2bearing no intramolecular cycle did not emit fluorescence in solution, cis ‐TPE dicycles 3– 5displayed a strong emission in solution. In THF, Φ fof 2was 0% but that of 3and 4was 24 and 49%, respectively. Due to the bridge, p ‐phenylenedimethyl units were short and rigid, and the propeller‐like conformation of the TPE dicycle should have been fixed. However, the enantiomers obtained by chiral high‐pressure liquid chromatography (HPLC) rapidly racemized in solution at room temperature. This result indicated that the phenyl rings of the cis ‐TPE dicycles was still able to rotate although the rotation had a little restriction. In addition, the formaldehyde groups of cis ‐TPE dicycle 3reacted with chiral α‐methylbenzylamine to form imine, and positive circular dichroism (CD) signals could be induced by ( R )‐α‐methylbenzylamine but negative CD signals were aroused by ( S )‐α‐methylbenzylamine. The CD signals should result from a single‐handed propeller‐like conformation induced by the enantiomer of the chiral amine because neither individual 3nor amine enantiomers could show such signals. This meant that the propeller‐like conformation of 3could be transformed from the left‐handed helical direction to the right‐handed helical direction, demonstrating that the phenyl could freely rotate. Consequently, it could be inferred that about 25–50% Φ fshould come from the RDBR process (see Figure 3.16).
As expected, the resolved enantiomers from TPE tetracycle 6were stable due to complete immobilization of its propeller‐like conformation. The racemate of 6had Φ fup to 97%, and both of the two enantiomers had a quantitative Φ fup to 100% due to restriction of not only double bond rotation but also phenyl ring rotation. Compared with the corresponding TPE dicycle 4, TPE tetracycle 6showed a twofold increase in fluorescence intensity, demonstrating that RDBR and RIR play equal key roles on the AIE effect.
In addition, the two enantiomers of 6, M ‐ 6(left‐handed helical propeller‐like configuration) and P ‐ 6(left‐handed helical one), emitted strong circularly polarized luminescence (CPL) signals in THF with a dissymmetric factor ( g lum) of +3.1 × 10 −3for M ‐ 6and −3.3 × 10 −3for P ‐ 6, indicating that the propeller‐like conformation of TPE was maintained even at the excited state. Moreover, the | g lum| of CPL was similar with the g absof absorbance ( g abs= 2(Δ ε / ε )) of 2.4 × 10 −3for M ‐ 6and P ‐ 6, indicating little conformational change between the ground state and excited state. This further confirmed that no double bond rotation occurred for this TPE tetracycle (see Figure 3.17).
Читать дальше
















