During the course of luminescence research over the past decades, people have found that there is a deleterious photophysical phenomenon called concentration quenching (CQ): luminescence of solution becomes dimming and even quenching when concentration is increasing. It has gradually been identified that the quenching of luminescence is caused by the aggregation of luminescence molecules, referred to as “aggregation‐caused quenching” (ACQ). Aromatic hydrocarbon luminophores, due to their high modifiability and diversity, have always been the most studied luminescence materials. However, the ACQ effect is especially obvious in aromatic hydrocarbons and their derivatives [1]. Taking fluorescein as an example (see Figure 3.1, upper panel) [2], the dilute solution of fluorescein in water has a bright green emission but the fluorescence of the solution gradually diminishes and eventually disappears after acetone fraction is increased and aggregates are formed. This ACQ effect is ascribed to the close π – π stacking interactions between fluorescein molecules in aggregated state due to planar structure of the polycyclic aromatic hydrocarbon. While these planar luminophores are beneficial for application in solution, they suffer from severe limitation in applications that must be carried out in aggregated state, such as biosensor [3] and organic light‐emitting diode (OLED) [4], which are usually used in solid state.

Figure 3.1 Fluorescence photographs of solutions and suspensions of (upper panel) fluorescein (15 μM) in water/acetone mixtures with different fractions of acetone ( f a) and (lower panel) hexaphenylsilole (HPS; 20 μM) in THF/water mixtures with different fractions of water ( f w).
Source: Reproduced with permission from Ref. [2]. Copyright 2014, WILEY‐VCH Verlag GmbH & Co. KGaA.
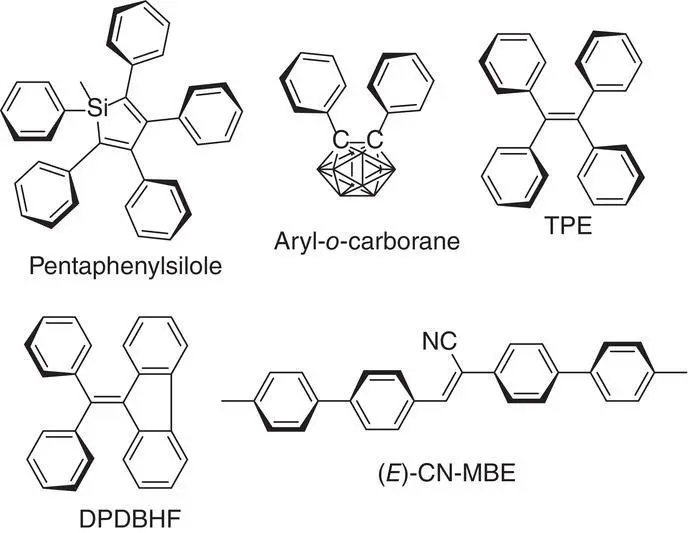
Figure 3.2 Representative AIEgens, including pentaphenylsilole, tetraphenylethylene (TPE), aryl‐o‐carborane, 1‐cyano‐1,2‐bis(4′‐methylbiphenyl)ethylene (CN‐MBE), and diphenyl dibenzofulvene (DPDBF).
Many efforts have been made to mitigate the effects of the ACQ, but few have succeeded until 2001 [5]. In this year, Tang et al. discovered an uncommon luminophore, 1‐methyl‐1,2,3,4,5‐pentaphenylsilole, which conducted no emission in dilute solution but emitted strong fluorescence in aggregated state. They coined a new phenomenon as aggregation‐induced emission (AIE), which is opposite to the ACQ phenomenon. Since then, a wide variety of molecules such as hexaphenylsilole (HPS) [6], tetraphenylethylene (TPE) [7], 1‐cyano‐1,2‐bis (4'‐methylbiphenyl)ethylene (CN‐MBE) [8], and aryl‐o‐carborane [9] (see Figure 3.2) have been discovered to have AIE properties. These AIE luminogenes are also shortened as AIEgens afterwards. Taking HPS as an example, it is nonemissive when its molecules are dissolved in a good solvent like THF, but its fluorescence turns on and becomes very strong after poor solvent like water was added and a suspension appeared (see Figure 3.1, lower panel). In contrary to the planar structure of the conventional luminophores, AIEgens often have a nonplanar and twisted structure, bearing crowded and anticoplanar aromatic rings. Due to the discovery of this new system and providing a better platform to study fluorescence in aggregated states, many research groups have been enthusiastically working on the practical application and decryption of AIE effect in the past two decades. A large number of magnificent achievements have been reached in various fields, ranging from solid emitter, bioimaging, chemosensing, optoelectronics to stimuli‐responsive systems [10–23].
To better guide the discovery and practical application of novel AIE molecules, an insight into the detailed luminescence process is highly needed. Up to date, although there are a large number of AIE mechanisms that have been put forward, the most popular and the most successful mechanism is the mechanism of restriction of intramolecular rotation (RIR) [12]. By this mechanism, it is known that the substituted groups such as aromatic rings of the AIEgens are able to freely rotate in solution so that the excited energy is released by the nonemissive rotation process and no or weak fluorescence light is emitted. In the aggregated state, the rotation of the substituted groups is restricted and the nonemissive rotation process is blocked. Therefore, the luminophore turns on its luminescence light and even displays strong emission. Later, RIR mechanism incorporates the process of the restriction of intramolecular vibrations (RIVs) and is developed into a mechanism of restriction of intramolecular motions (RIMs), which is applicable for wider AIE molecules [12–17].
However, many AIEgens such as TPE, α‐cyano‐stilbene, and their derivatives, which are most extensively studied as AIE molecules, bear a central double bond [12–17]. Under irradiation, their double bond may undergo rotation due to the homolytic cleavage of the π ‐bond at the excited state (see Figure 3.3). Direct and indirect evidence as well as theoretical calculation have demonstrated the restriction of the double bond rotation (RDBR), just like the restriction of the phenyl ring rotation, and play a key role on the AIE phenomena. Some researchers even consider that the RDBR mechanism has a major effect on the AIE effect. Therefore, RDBR is the important complement and enrichment of RIR mechanism. It further specifies the factors that affect the AIE phenomena and will be beneficial for exactly designing and improving novel AIE molecules. Therefore, we make a review on the RDBR mechanism of AIE phenomena in this chapter.
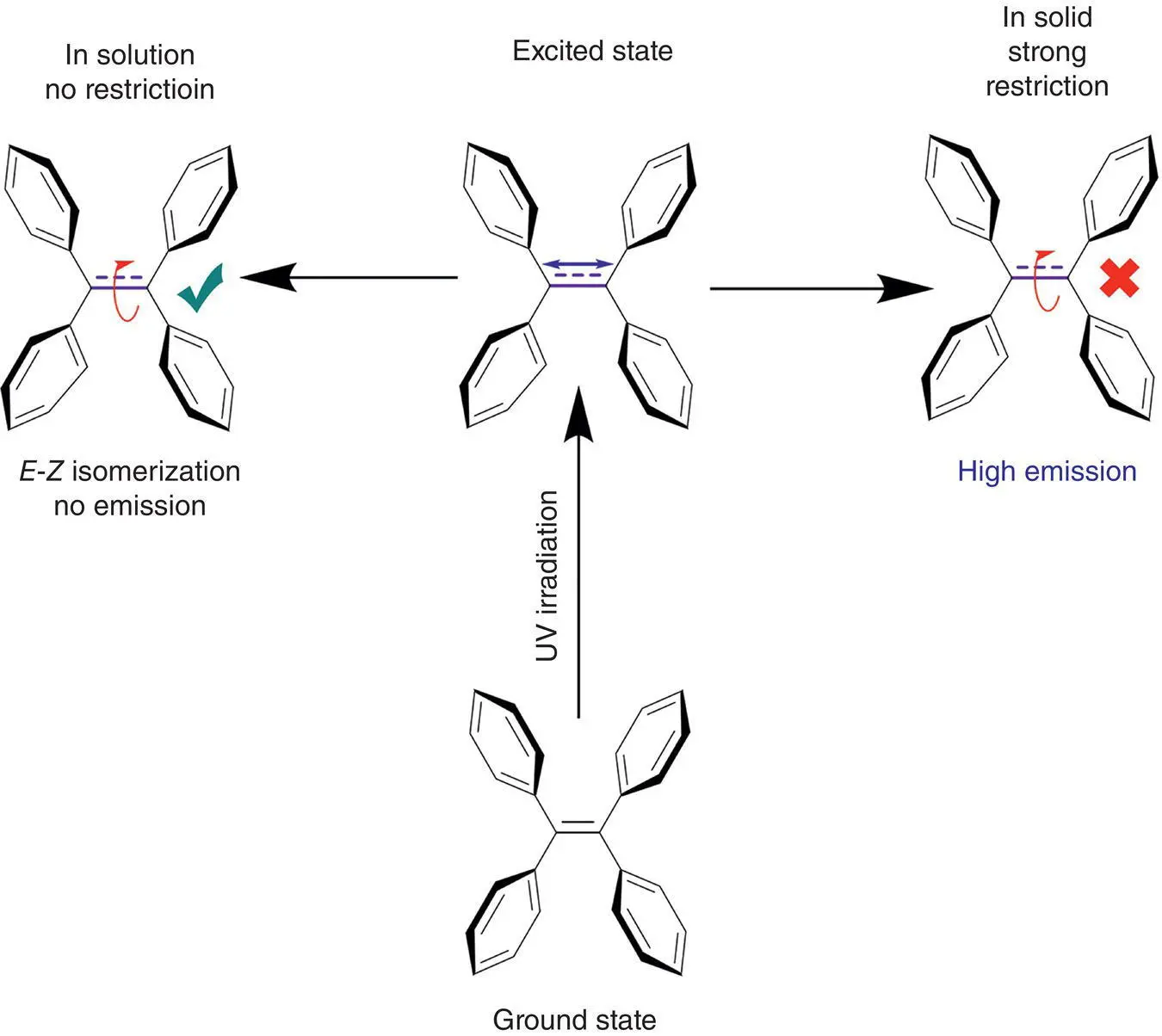
Figure 3.3 The rotation of a double bond at the excited state results in no fluorescence of TPE in solution, but in solid state, the rotation is blocked and strong emission turns on.
3.2 AIE Phenomena and Applications from RDBR Mechanism
3.2.1 Evolvement and Development of AIE Mechanisms
Since AIE concept is coined for the first time by Tang’s group in 2001, a large number of AIE molecules are reported. Accompanying the appearance of the AIE molecules in massive quantities, different kinds of AIE mechanisms have also been suggested. In general, when a planar luminophore forms an excimer after irradiation, the excited energy of one molecule will be transferred to other one and nonradiative decay occurs. Therefore, the fluorescence of the luminophore will be quenched in solid state. However, in 2007, Tao et al. [24] found that fluorenone derivatives 2,7‐dip‐tolyl‐fluorenone (DTFO) and 2,7‐bis(4‐( tert ‐butylthio) phenyl)‐fluorenone (DSFO) could form excimers but displayed AIE effect. Due to the formation of a dimer through hydrogen bonds in the crystal state, DSFO could give an excimer without arrangement adjustment after the dimer was excited. The excimer could decay back to the dimer, and the nonradiative decay pathways that existed in common excimers were greatly reduced, offering a strongly enhanced luminescence in the solid state (see Figure 3.4).
In 2004, Park et al. [25] reported that 1‐cyano‐ trans ‐1,2‐bis‐(3′,5′‐bis‐trifluoromethyl‐biphenyl) ethylene (CN‐TFMBE) could strongly gelatinate 1,2‐dichloroethane. The resultant gel emitted a strong fluorescence but the solution showed no fluorescence. They ascribed the AIE effect to the J‐aggregation of CN‐TFMBE molecules in solid state because a stronger red‐shift emission was observed in a mixed solvent of THF/H 2O 1 : 4 compared with the solution in THF. Meanwhile, the absorption band of the suspension in THF/H 2O 1 : 4 also displayed an obvious red shift with that of solution. But later, Ma et al. [26] found that the similar α‐cyano‐stilbene derivative CN‐DPDSB did not form compact π – π stacking in the crystal structure due to large cyano and phenyl groups. Instead, strong intermolecular cyano nitrogen–hydrogen interactions (2.43 and 2.56 Å) restricted the rotation of a double bond and a phenyl group in the solid state, causing its AIE effect (see Figure 3.5).
Читать дальше















