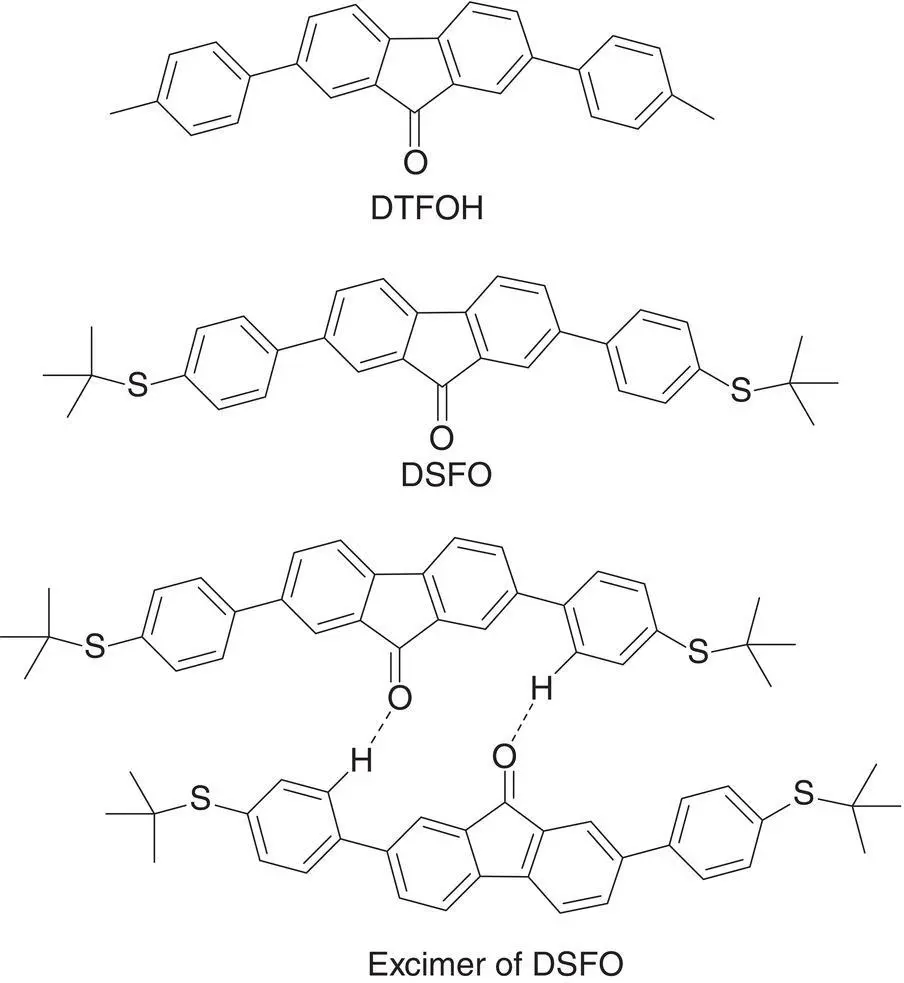
Figure 3.4 Structure of DTFO, DSFO, and excimer of DSFO.
Baumgartner et al. [27] synthesized a series of amphiphilic phosphonium compounds including DTP, which could form liquid crystalline and soft crystal phases in the solid state, emitting fluorescence 40 times stronger than its solution. It was found that DTP formed a folded conformation in solution in which the electron‐rich trialkoxybenzyl group was close to the electron‐deficient phospholium‐containing fused scaffold. In this closed structure, the photoinduced electron transfer (PET) process combined with phenyl rotation led to weak emission. In contrast, the solid DTP possessed an opened conformation with the trialkoxybenzyl ring pointed away from the main fused scaffold and the PET process disappeared. Therefore, DTP emitted a stronger fluorescence (see Figure 3.6).
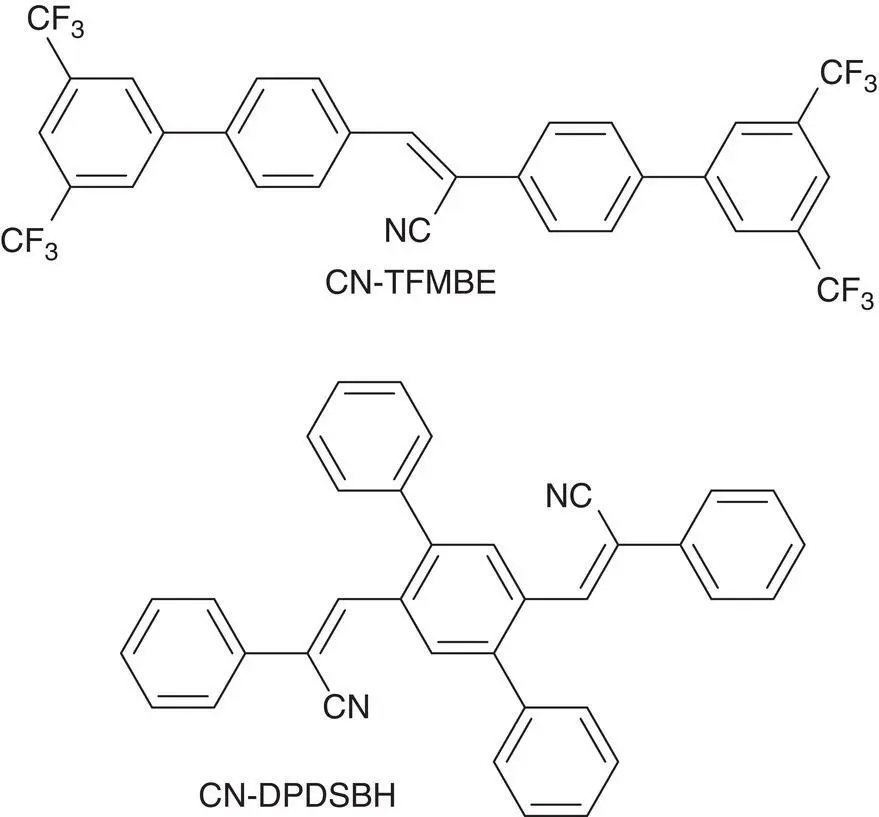
Figure 3.5 Structure of CN‐TFMBE and CN‐DPDSB.
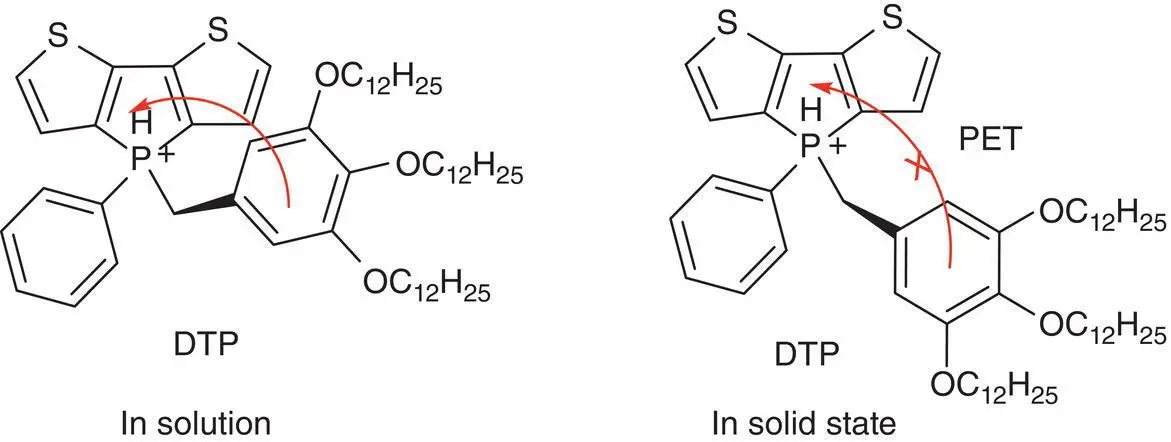
Figure 3.6 Structure of DTP and its PET process.
By cross‐dipole stacking instead of H‐aggregation, stilbene derivatives trans ‐DPDSB without a cyano substituent could emit a strong blue fluorescence in the crystal state. It is considered that the cross‐dipole stacking of the trans ‐DPDSB molecules can increase a finite transition dipole moment between the ground state and the lowest excited state of the clusters, promoting the light‐emission properties [28]. Tian et al. [29] also found that a butterfly‐like molecule 9,10‐bis(2,2‐diphenylvinyl) anthracene (BDPVA) formed cross‐dipole stacking in the crystal state and emitted a stronger fluorescence in solid than in solution (see Figure 3.7).
In addition, molecular planarization is also one of possible AIE mechanisms. Park et al. [8] ever reported that 1‐cyano‐ trans ‐1,2‐bis‐(4′‐methylbiphenyl)‐ethylene (CN‐MBE) possessed a twisted structure so that it emitted no fluorescence in solution. However, in solid state, the twisted structure was stretched out to give a more coplanar structure, which was helpful for its J‐aggregation and showed a strong emission (see Figure 3.8).
Very specially, Zheng et al. [30] recently found that a totally planar molecule 5,7,12,14‐tetraoxapentacene (TOP) also displays the typical AIE effect. In highly polar solvent, TOP had no emission due to the solvation role but emitted a strong fluorescence in solid state because of the largely slipped π – π stacking. Although this molecule is planar, oxygen atoms on the different molecular planes prevented cofacial π – π stacking that can arouse ACQ result because of the repulsive force between oxygen atoms (see Figure 3.9).
Although these above AIE mechanisms can well explain the emission or emission enhancement of some special luminophores in solid state, they are not applicable for more extensive and typical AIEgens such as TPE and phenylsilole derivatives until the RIR mechanism was put forward by Tang et al. [12–17]. The RIR mechanism was first proposed to explain the AIE effect of silole derivatives such as HPS (see Figure 3.10). HPS is a typical AIE molecule. When HPS molecules are dissolved in a good solvent at low concentrations, fluorescent quantum yield of the solution is extremely low ( Φ f∼ 0.1%). However, the emission is dramatically enhanced after the fraction ( f w) of poor solvent such that water reaches ∼50 vol % and reaches a maximum of ∼22% at f w= 90 vol%, being approximately 220‐fold increase in fluorescence quantum yield. As shown in the molecular structure of HPS, the central silole is connected with six phenyl rings by rotatable single bonds, which make HPS to show a great conformational flexibility in isolated phase. Due to the six phenyl rings around a tiny core, the HPS molecular structure is rather distorted. This torsion makes a great tension between adjacent phenyl rings, and it is difficult for the entire molecule to form a planar conformation. It can be seen from the crystal structure that HPS molecules possess a propeller‐like conformation and each phenyl rings exhibit different degrees of distortion compared with the silole core. This nonplanar structure makes it difficult for molecules to pack tightly. Therefore, no π − π stacking interaction is observed for HPS in the solid state, avoiding the ACQ effect. Meanwhile, the dynamical rotation of six phenyl rings is restricted in the solid state and the nonradiative channel is blocked. Therefore, in aggregates, high emission is accessible.
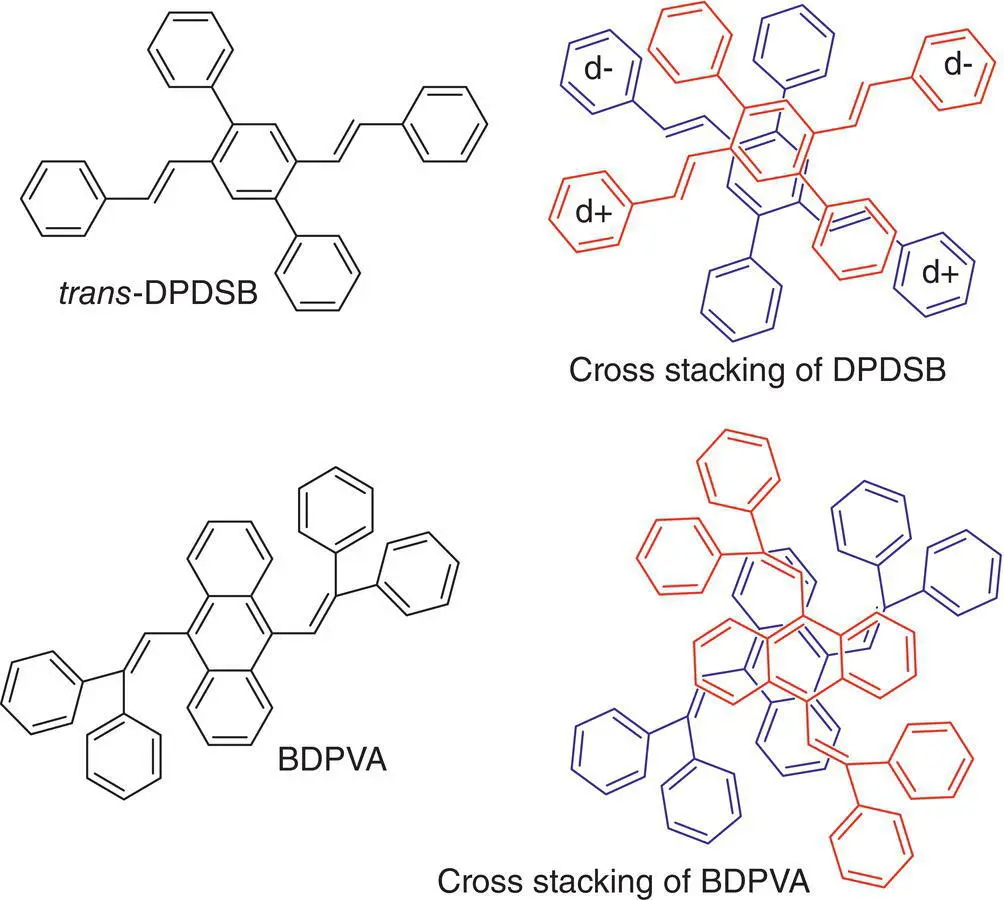
Figure 3.7 Structure of DPDSB and BDPVA and their cross‐dipole stacking.
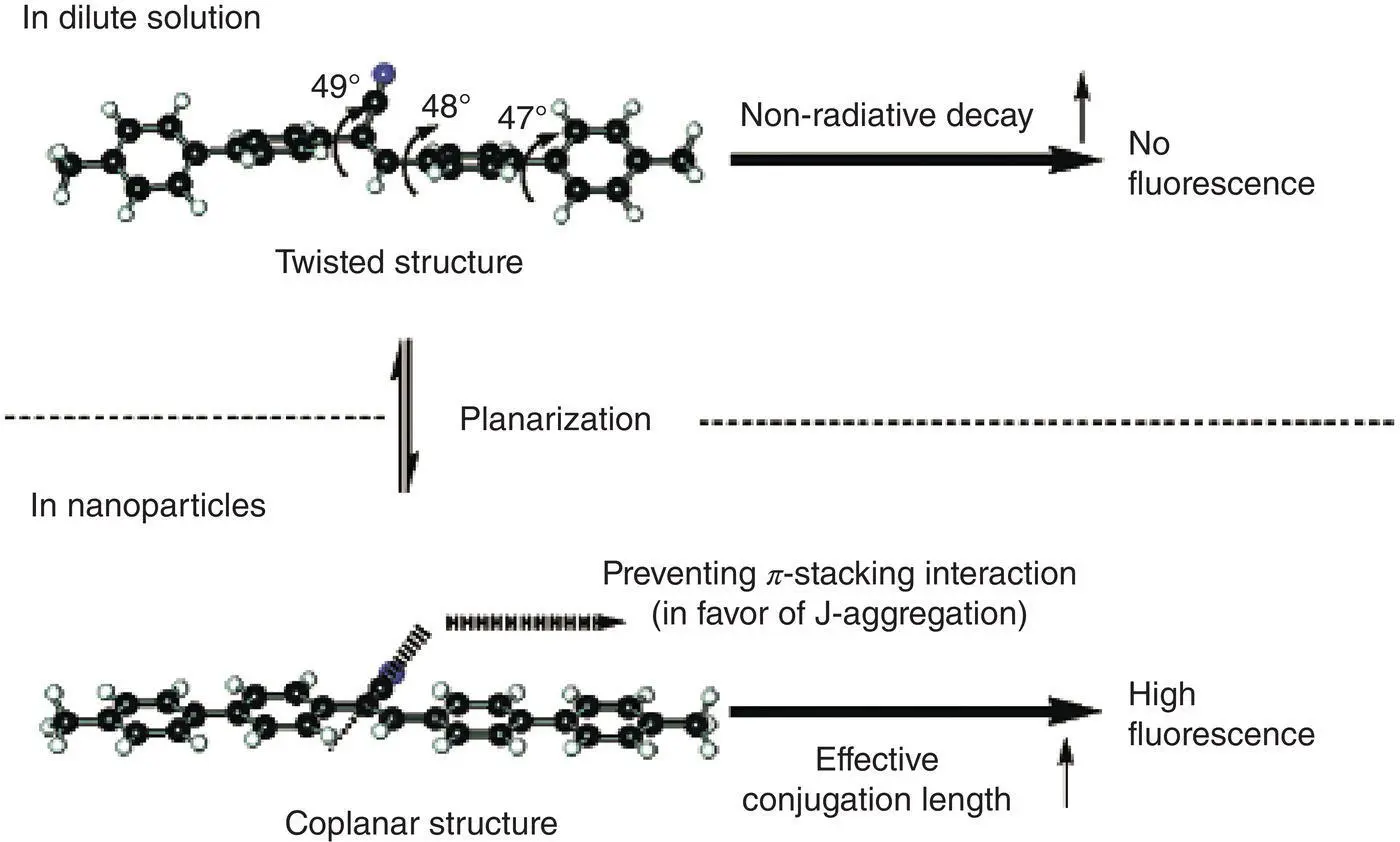
Figure 3.8 Emission of CN‐MBE in the solid state by molecular coplanarization.
Source: Reproduced with permission from Ref [8]. Copyright 2002, American Chemical Society.
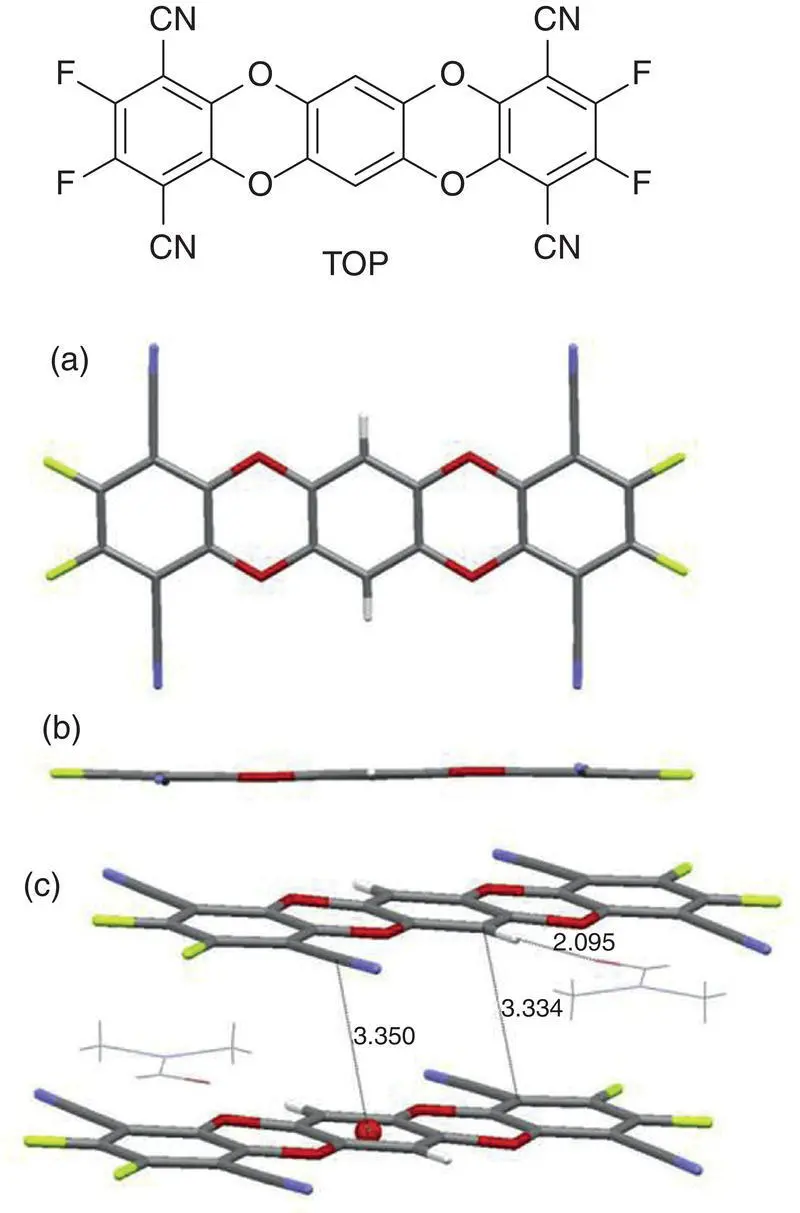
Figure 3.9 Molecular structure and crystal structure of TOP viewed (a) perpendicular to the molecular plane and (b) along the molecular plane; (c) the slipped π – π packing of the molecules.
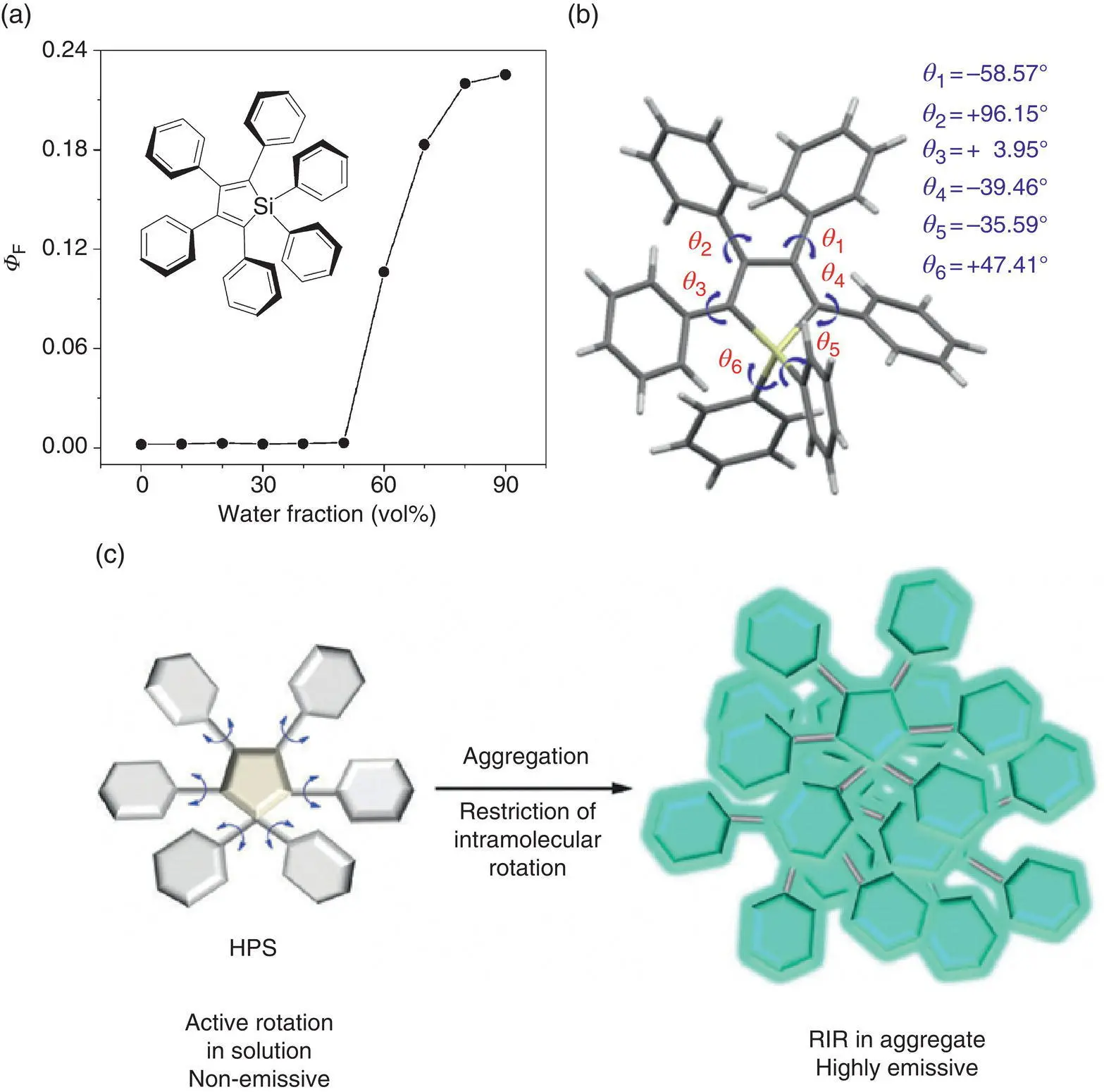
Figure 3.10 (a) Fluorescence quantum yield of HPS vs water fraction in acetone/water.
Source: Reproduced with permission from Ref. [11]. Copyright 2003, American Chemical Society.
(b) Molecular conformation of HPS.
Source: Reproduced with permission from Ref. [12]. Copyright 2015, American Chemical Society.
(c) HPS molecule emission.
Source: Reproduced with permission from Ref. [15]. Copyright 2015, Royal Society of Chemistry.
However, it is found that some luminophores lack substituents that can rotate but display typical AIE phenomenon. For example, annulenylidene THBA (see Figure 3.11a), whose phenyl rings are fixed by a pair of ethylene tethers, still exhibits strong emission in aggregates but no emission in dilute solution [12, 31, 32]. Consequently, the RIV mechanism is raised. For this class of luminophores, intramolecular vibration instead of rotation is restricted in the aggregated state, which turns on the luminescence. Recently, Tang et al. reported cyclooctatetrathiophene (COTh) and its derivatives that are AIE active [33]. There are no rotatable unit in the molecular structure of COTh (see Figure 3.11b) in both ground and excited states. However, in solution, the intramolecular vibration of excited state could cause up‐down conformation inversion and result in a fluorescent quenching. Because of the RIV mechanism, COTh emits a strong green fluorescence in the solid state.
Читать дальше



















