If an AIEgen includes both rotating and vibrating units, obviously, neither RIR nor RIV can explain its AIE effect alone. Therefore, RIM mechanism is used to explain AIEgens with more complex structures and emission units. Some examples of such AIEgens are shown in Figure 3.12Those molecules are suitable for the RIM mechanism because of their multiform motions of isolated molecules [34–37]. RIM mechanism advocates that the fluorescent quenching of AIEgens in dilute solution results from various ways of intramolecular motions including twisting, deforming, flapping and probably motion of solvating molecules in addition to rotation and vibration. While it greatly broadens the scope of application of the AIEgens, it also poses new challenges for researchers. Because, under real conditions, in order to better guide the optimization of the structure of AIEgens, people need to know the exact factor of motions, rather than simple and general motions. Consequently, although the RIM mechanism is the most popular and applicable, a lot of detail contents need to be embodied and supplied.
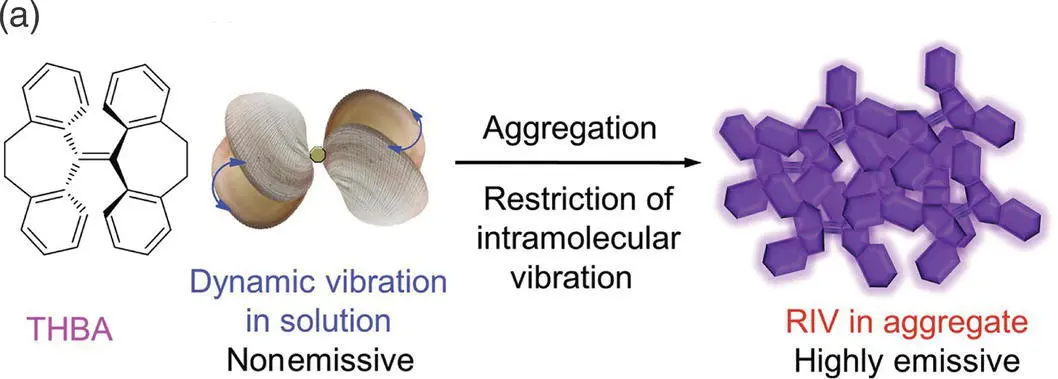
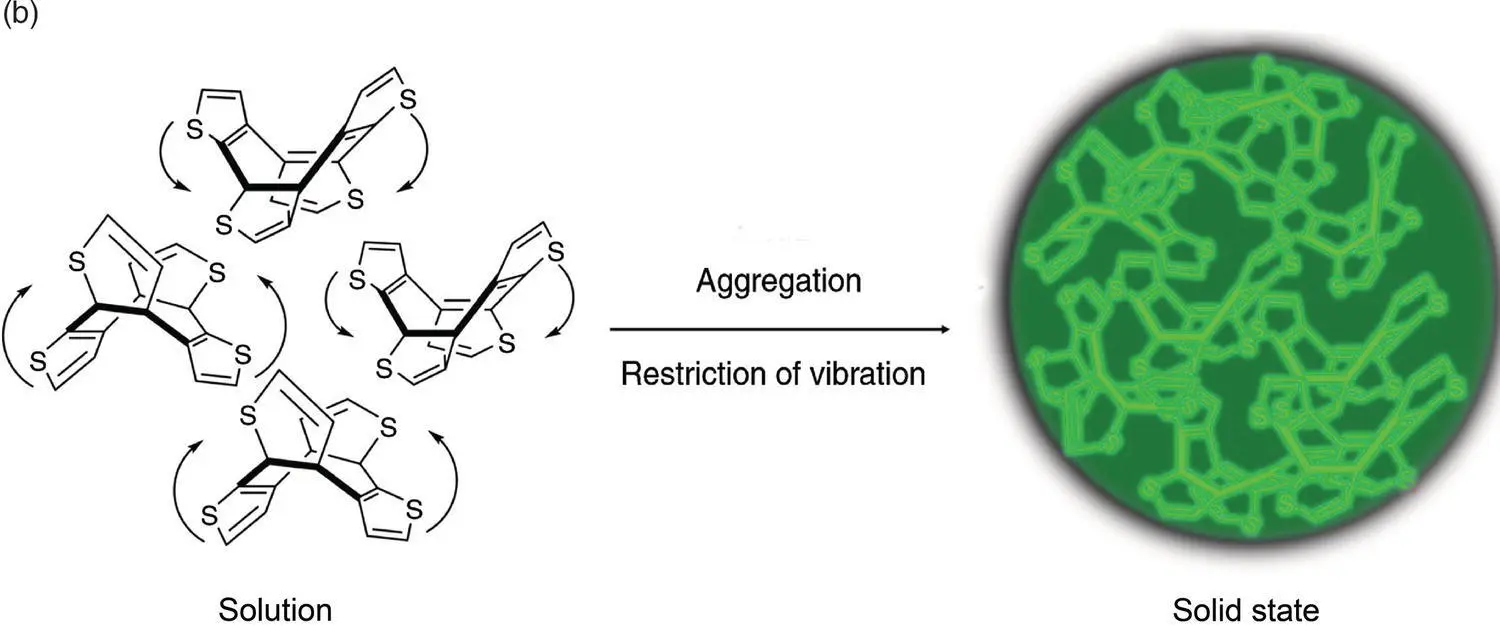
Figure 3.11 (a) RIV mechanism of THBA.
Source: Reproduced with permission from Ref. [12]. Copyright 2014, WILEY‐VCH Verlag GmbH & Co. KGaA.
(b) RIV mechanism of COTh.
Source: Reproduced with permission from Ref. [33]. Copyright 2019, Springer Nature.
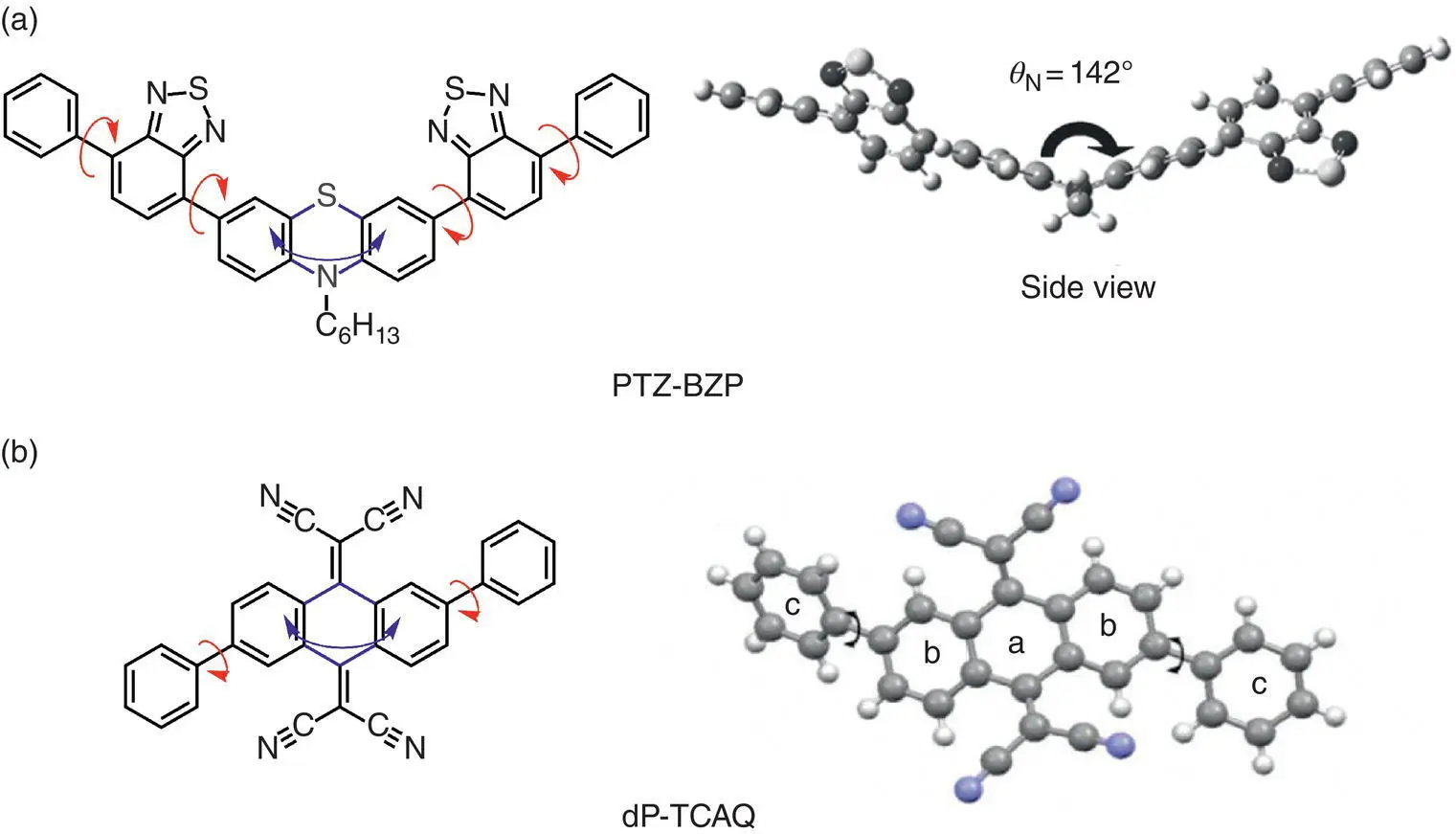
Figure 3.12 Examples of luminogens whose AIE activities are ascribed to the process of restriction of intramolecular motions (RIMs). (a) Molecular structure of PTZ‐BZP and DFT‐optimized geometry of a simplified structure with a methyl substituent.
Source: Reproduced with permission Ref. [34]. Copyright 2014, WILEY‐VCH Verlag GmbH & Co. KGaA.
(b) Molecular structure and single‐molecular structure of dP‐TCAQ.
Source: Reproduced with permission from Ref. [35]. Copyright 2013, Royal Society of Chemistry.
3.2.2 Investigation of RDBR AIE Mechanism by E / Z isomerization
It is known in the textbook of organic chemistry that a carbon–carbon double bond is unable to rotate in general conditions. However, under irradiation or at high temperature, the π ‐bond of the double bond can undergo homolytic cleavage and the double bond can rotate. The most well‐known example is molecular photoswitches or even molecular motors in which the Z ‐isomer and E ‐one can be reversibly transformed into each other ( E / Z isomerization, EZI) through double bond rotation under irradiation or heating [38, 39]. Among AIEgens, most of them such as TPE, cyanostilbene, and their derivatives possess one essential double bond. Whether the double bond undergoes a rotation under irradiation of ultraviolet (UV) light and arouses nonradiative decay of these AIEgens in solution is brought to concern even from the moment the AIEgens start to appear. Because of the relatively simple synthesis method, easy to modify, and stable AIE effect, TPE and derivatives become the most extensively studied AIEgens. Meanwhile, RIR mechanism is rightly concluded from the investigation of TPE and its derivatives. Whether the double bond rotation of the TPE unit is involved in the AIE process is always in controversy. Due to the symmetrical structure of TPE, it is difficult to observe the obvious effect of double bond rotation without proper modification. In the beginning, it was thought that the double bond had little effect on the AIE mechanism.
In 2012, Tang et al. prepared a dimethylated TPE derivative called 1,2‐diphenyl‐1,2‐di( p ‐tolyl)ethylene (DPDTE) [40]. To conduct an EZI experiment, a pure E or Z conformation is needed. Therefore, they used an unconventional way, a Pt‐catalyzed reaction rather than the normal McMurry coupling reaction, to provide a Z ‐rich product. In 1H NMR spectra, E ‐isomer of DPDTE has two classes of protons that appear at δ 6.89 and 2.24 ppm, while the corresponding protons have signals at δ 6.91 and 2.26 for the Z ‐isomer. By comparing the integral areas of these two protons of the two isomers, it is revealed that the as‐prepared product is Z ‐rich and has a Z / E ratio of 93 : 7. Through recrystallization, pure Z ‐isomer was obtained. By using a UV lamp of spectrofluorometer as a light source to irradiate a solution of Z ‐DPDTE for a long period of time (29 minutes), they hoped to get EZI result. However, throughout the whole radiation process, no change of 1H NMR spectra could be observed. But when they used a UV lamp with a much stronger power to irradiate the DPDTE solution, EZI happened in a much shorter period. The NMR spectra showed that the peaks of E ‐isomer were raised after the strong UV irradiation. In the same year, they reported similar results of TPE derivative named BPHTATPE [41]. By attaching large and polar moieties to a TPE core, they prepared BPHTATPE whose difference in the shape and polarity between E ‐ and Z ‐isomers can allow these two isomers to be separated just by making use of simple column chromatography. They performed EZI experiments on both the two isomers. It turned out that BPHTATPE could undergo E / Z isomerization only under a powerful UV light and high temperature (>200 °C) (see Figure 3.13). Consequently, they deduced that the EZI was not involved in its AIE process under the normal photoluminescence spectral measurement conditions.
But in 2016, the possible involvement of a double bond rotation in the AIE process of the TPE‐based luminogen was found by Tang et al. [42] They designed a TPE derivative 1,2‐bis(4‐fluorophenyl)‐1,2‐bis(4‐methoxyphenyl) ethylene (TPE‐FM), which possessed more polarity due to the strong electron‐attracting fluorine atoms and the electron‐donating methoxyl groups (see Figure 3.14). Therefore, the E ‐ and Z ‐isomers are easily separated and single crystals even grow well due to the large molecular polarity. The obtained crystal structure can make sure the identity of E ‐ and Z ‐isomers. With the identified isomers in hand, they carried out more careful test on the EZI of TPE‐FM in solution. Due to the difference of 1H NMR spectra of E ‐ and Z ‐isomers, the EZI process of TPE‐FM was observed in the solution at a high concentration of 10 mM under a UV radiation of high power (321 nm, ~0.47 mW/cm 2) for a 15‐minutes irradiation. But emission spectrum was generally measured at a highly diluted solution (~10 μM). The EZI process at high concentration did not guarantee its occurrence at highly diluted solution. Considering the low sensitivity of 1H NMR spectroscopy and unable to measure NMR spectra at very low concentration, they prepared three cuvettes of solutions (10 μM), which were irradiated by a 312‐nm UV light from the xenon lamp with a low power (321 nm, ~0.012 mW/cm 2) in the spectrofluorometer for specific periods of time. The solutions from the three cuvettes were combined and evaporated to dryness in a dark room. The obtained solid was dissolved in a small amount of dichloromethane‐d 2for NMR measurement. By this effort, 1H NMR spectra with high quality showed that EZI truly occurred even at very low concentration under low‐power excitation. Whereas it is difficult to determine whether the double bond rotation played a major role or minor role on the emission process of the TPE AIEgens, compared with the phenyl ring rotation.
Читать дальше















