Recently, Zheng et al. [45] have exploited the RDBR mechanism to improve the sensitivity of DNA detection and enhance the chiroptical properties from TPE AIEgens. In this regard, cis ‐TPE macrocycle diquaternary ammoniums 11were designed and synthesized. As a comparison, gem ‐isomers 12were also prepared. As soon as the two ammonium arms at the cis ‐position simultaneously hold on one DNA chain by electrostatic attraction, the formed cycle together with the original cycle will completely immobilize the double bond rotation at the excited state and arouse the enhancement of the AIE effect (see Figure 3.20).
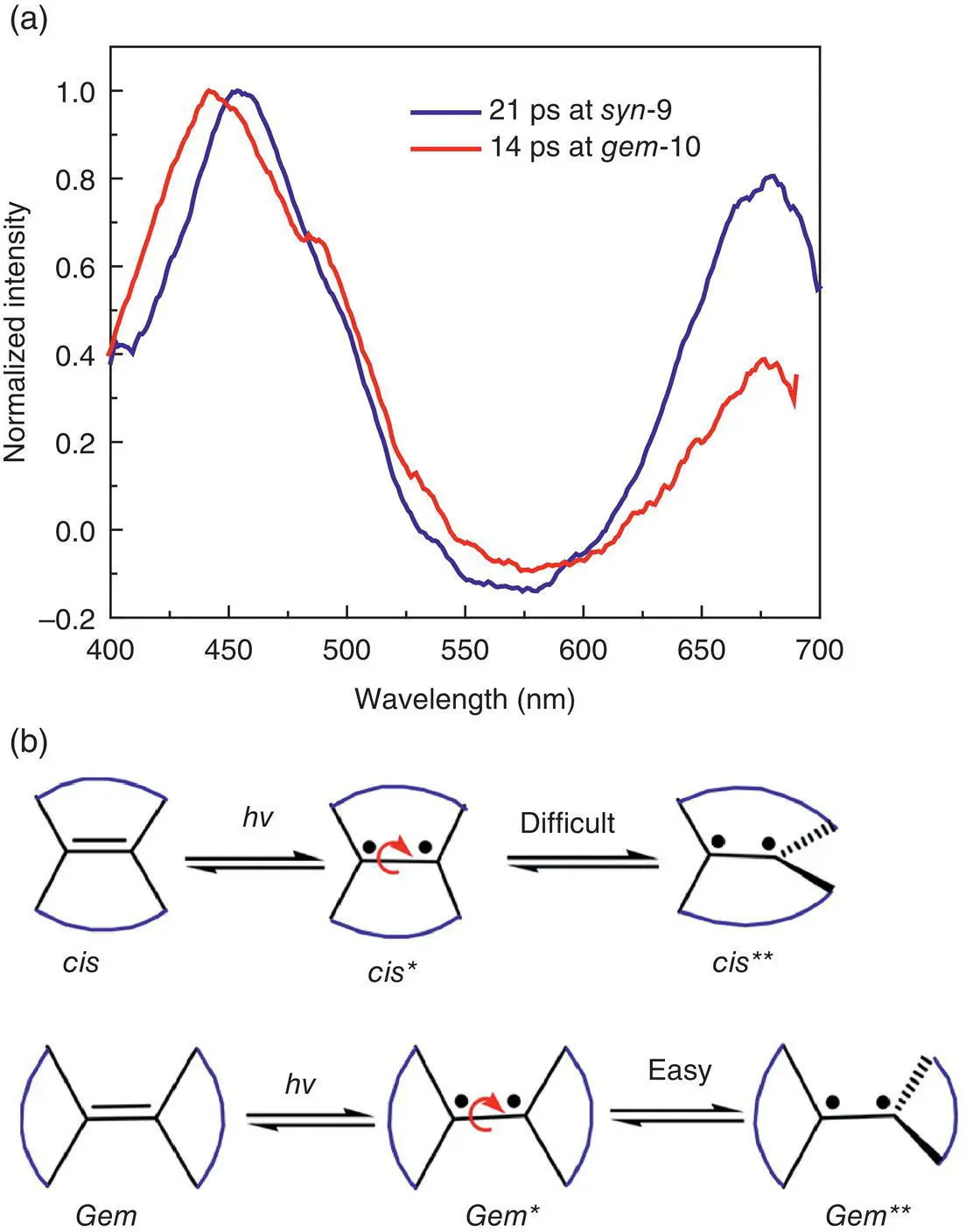
Figure 3.19 (a) Femtosecond transient absorption spectra of 9and 10at the respective maximum intensity. (b) Diagrammatic sketch of the normal excited state ( cis *and gem *) and twisted excited state ( cis **and gem **) of the double bond.
Source: Reproduced with permission from Ref. [44]. Copyright 2018, American Chemical Society.
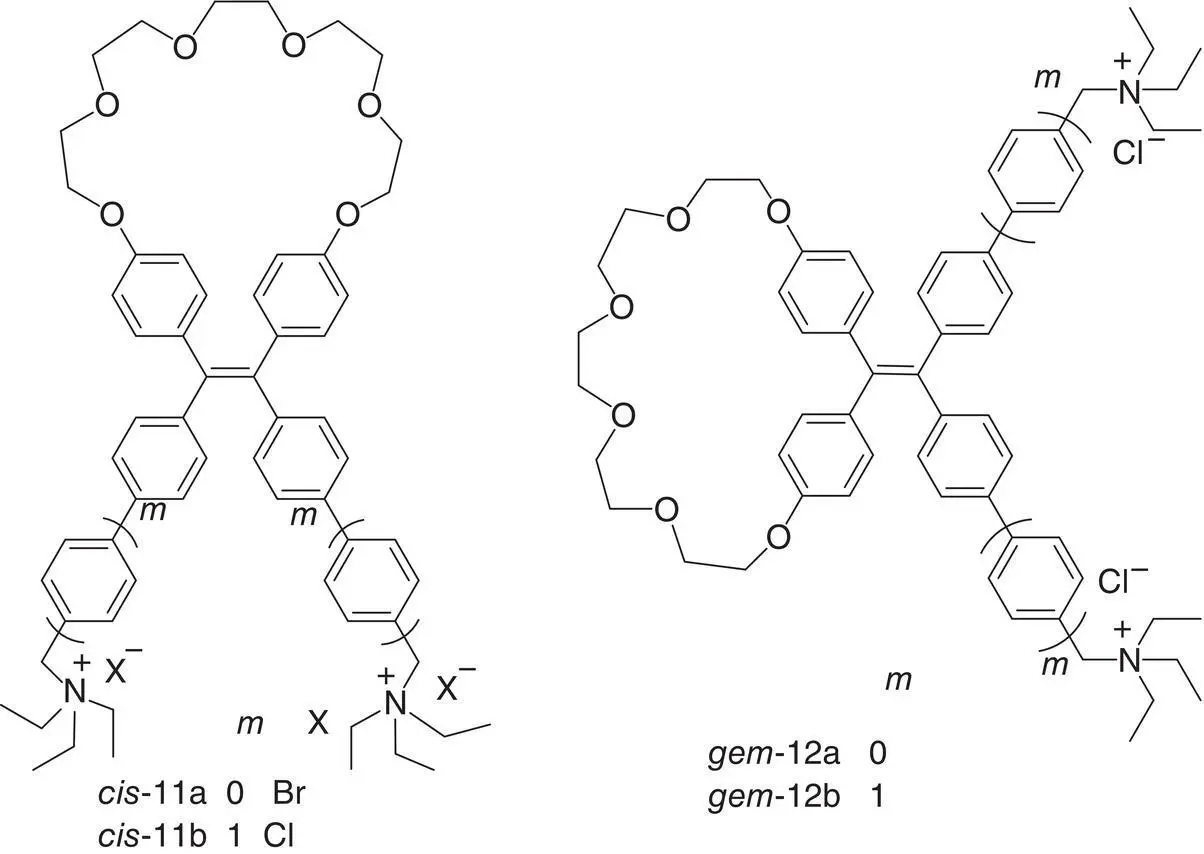
Figure 3.20 Structures of cis ‐ and gem ‐TPE macrocycle diquaternary ammoniums 11and 12.
Due to the limitation of crown ether cycle, both cis ‐ 11and gem ‐ 12display weak emission in solution. However, while the quantum yield of gem ‐isomer 12was 1.5%, the cis ‐TPE ammonium 11had a Φ fof 3.0%, which was a 2.0‐fold stronger than that of the gem ‐one. This should be ascribed to the partial limitation of the double bond rotation in cis ‐isomers but no restriction of the double bond rotation in the gem ‐one. When cis ‐TPE ammonium cis ‐11aor cis ‐11bwas added to the solution of calf thymus DNA (CT‐DNA), strong new CD signals were induced. In addition to CD signals of DNA itself at short wavelengths, one bisignate band from about 370 nm (+) to 310 nm (−), which should be ascribed to the single‐handed propeller‐like conformation of the TPE unit, appeared. Conversely, gem ‐isomers gem ‐12aand gem ‐12bshowed weak CD signals from the TPE unit and did not form a new bisignate band. Probably because of more flexibility of the TPE unit in the gem ‐isomer than in the cis ‐one, DNA was unable to induce the stable single‐handed propeller‐like conformation.
Outstandingly, strong CPL emission was observed in the drop‐cast film from a mixture of cis ‐11aand cis ‐11bwith CT‐DNA in water, while the gem ‐isomer–CT‐DNA film emitted no CPL signals. The CPL dissymmetric factor ( g lum) of 0.0028 and 0.016 for cis ‐11aand cis ‐11b, respectively, was much larger than that from the mixture of DNA with other AIEgens. Even in solution, strong CPL light was emitted from a mixture of cis ‐isomer with CT‐DNA in water but no CPL signals were found for the mixture of gem ‐isomers with CT‐DNA. With fish sperm DNA (FS‐DNA), a similar result was obtained. Considering the structure of the cis ‐isomers, the CPL enhancement should result from the more RDBR process of cis ‐isomers in which the formed cycle in situ together with the original cycle in the cis ‐position firmly restricts the double bond rotation not only at the ground state but also at the excited state (see Figure 3.21).
Given that the obvious interaction of the TPE diammoniums with DNA, they should be excellent sensor for the detection of DNA. It was truly that the fluorescence of TPE macrocycle diammoniums in water was increased when FS‐DNA was added into the solution at 1.0 × 10 −6M. But the solution from a mixture of cis ‐isomer with FS‐DNA showed stronger fluorescence than that from the corresponding gem ‐isomer and FS‐DNA. The fluorescence intensity was linearly increased in the range of DNA concentration less than 1.0 × 10 −8M. As a result, the detection limit for DNA analysis was obtained. It was found that the detection limits were 123, 74, 496, and 235 pM for 11a, 11b, 12a, and 12b, respectively. The cis ‐isomers had always much lower detection limit than the gem ‐ones. And the detection limitation of 74 pM from cis ‐isomer is among the best results from AIE DNA sensors. The higher sensitivity of cis ‐isomers than that of gem ‐isomers should also come from the RDBR mechanism. As shown in Figure 3.22, the restriction of a double bond of cis ‐isomers upon binding to DNA chain enhanced the AIE effect. Therefore, the sensitivity was significantly increased.
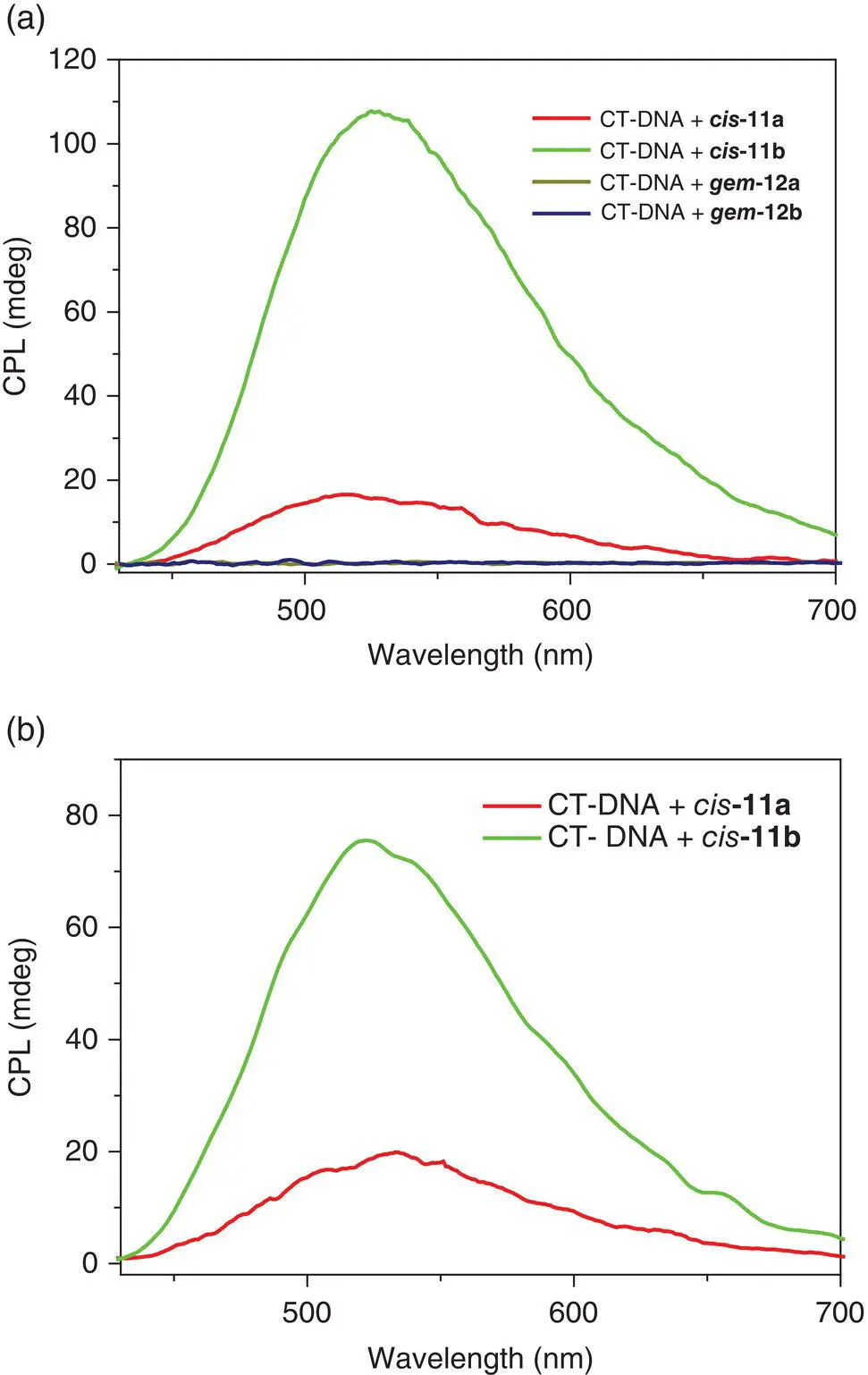
Figure 3.21 CPL spectra of a drop‐cast film (a) and solution (b) from a mixture of cis ‐TPE isomers and gem ‐TPE isomers with CT‐DNA in water.
When the cis ‐TPE macrocycle diammonium was synthesized using octaethylene glycol as a bridge, the resultant crown ether cycle is large enough to allow the EZI. As shown in Figure 3.23, the as‐prepared cis ‐ 13could be converted into trans ‐ 13under an irradiation of a 365‐nm portable UV lamp both in organic solvent and in water. Under a 365‐nm light from one fluorophotometer, the absorption spectrum of cis ‐ 13in CH 2Cl 2had a gradual absorbance increase at 355 and 278 nm but a constant decrease at 315 nm with irradiation time, and showed an obvious isosbestic point at 336 and 302 nm, indicating the conversion from cis ‐isomer to trans ‐one. In CDCl 3, after irradiation by a 365‐nm portable UV lamp for one hour, another set of signals appeared beside signals of cis ‐ 13in the 1H NMR spectrum. Especially, besides the signals of the aromatic proton near to the oxygen atom (6.71 ppm, double), benzyl methylene (4.82 ppm, single), and the ethylene proton close to the aromatic ring (4.09 ppm, triple), new peaks that were well separated and had the same shape and split with that of cis ‐isomer appeared at a lower field. The integral area of these new peaks was all almost equal to that of cis ‐ 13, suggesting a 50% conversion of cis ‐isomer to trans ‐one. In DMSO, the converted trans ‐isomer (about 20%) by light could completely come back to cis ‐isomer under heating at 180 °C. This result suggested that the double bond of the TPE derivatives was easy to rotate in the excited state.
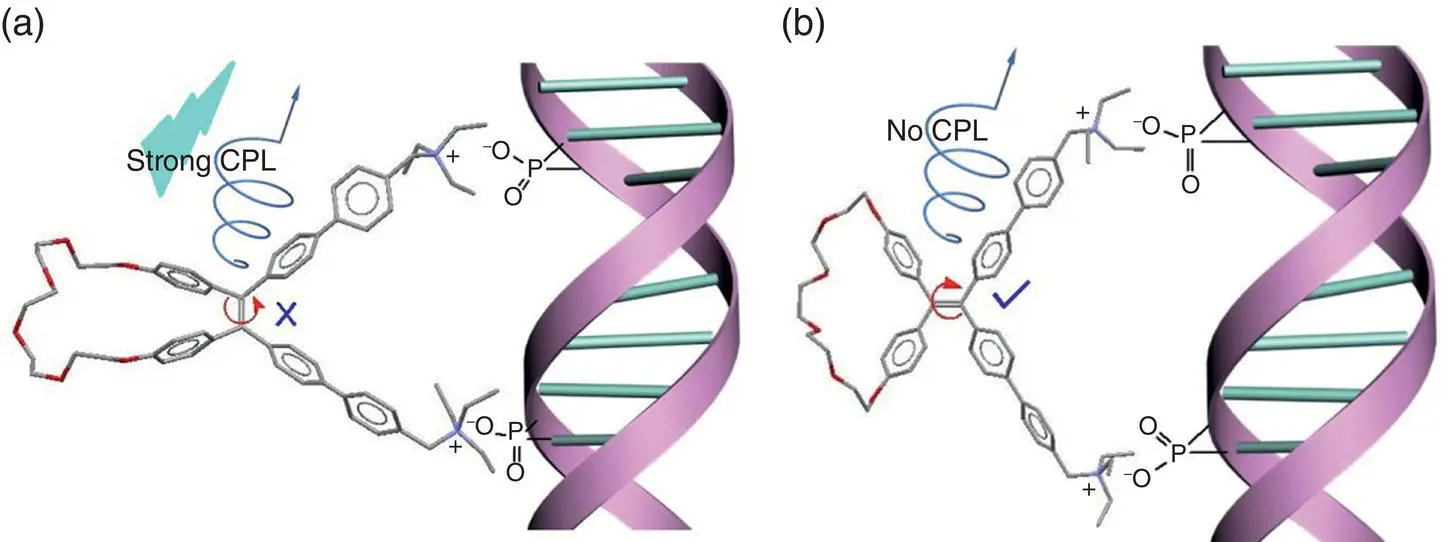
Figure 3.22 Schematic diagram of the binding of TPE cycle diammoniums 11(a) and 12(b) to the DNA strand.
Читать дальше
















