Source: Reproduced with permission from Ref. [61]. Copyright 2018, American Chemical Society.
Due to steric hindrance from the close stacking, it was energetically demanding for the rotation around the ethylenic bond in order to reach the S 0/S 1CIs when the ( E )‐CN‐MBE crystal was excited. Therefore, high emission is permitted in the solid phase. However, because of the loosely packed structure that allowed for the rotation around the ethylenic bond to reach the S 0/S 1CIs, ( Z )‐CNMBE did not exhibit fluorescence. Obviously, the restriction of the rotation of a double bond of ( E )‐CN‐MBE is crucial for its emission in aggregates.
Kimizuka et al. [62] demonstrated aggregation‐induced photon upconversion (iPUC) based on control of the triplet energy landscape. Using AIEgen (2Z,2′Z)‐2,2′‐(1,4‐phenylene) bis(3‐phenylacrylonitrile) (PPAN) (see Figure 3.36) as an acceptor and Pt IIoctaethylporphyrin (PtOEP) as a donor, when triplet states of acceptor were populated by a triplet sensitizer in solution, the TTA‐UC emission was not observed. In contrast, crystalline powder samples displayed a clear UC emission. For explaining such phenomena, the structure on the ground state (S 0) and the excited triplet state (T 1) both in solution and in the crystal was simulated. It was revealed that in solution, the double bond was dramatically twisted and stretched from S 0minto T 1min, resulting in the barrier‐free T 1‐to‐S 0intersystem crossing (ISC). And eventually, no fluorescence was observed. However, in the rigid crystalline state, this conformational‐twisting‐driven transition was effectively prohibited.
Gierschner et al. [63] prepared a series of dicyano‐distyrilbenzene (referred to DCS) derivatives with two different CN substitution patterns (α and β in Figure 3.37). The α‐series compounds were AIE‐active, but the β‐series were radiative in both solution and crystal states except for β‐DCS. Evidently, this phenomenon contradicted to the principal of RIR; hence, computational studies were carried out to inspect the main reason for the difference between α‐ and β‐series.
The TDDFT and CASSCF calculation results showed that there was a CI between S 1and S 0for each compound in CHCl 3when the double bond twisted 90° ( φ DB= 90°; see Figure 3.38). The energy of CI was identical for both α‐ and β‐series (2.78 eV), but the barrier was different for them to reach CI. In α‐series, due to the energies of initially excited FC ( E FC), the structure range from 2.9 to 3.2 eV was higher than that of CI, making the CI available for nonradiative decay. In β‐series, the energies of E FCare lower than CI, making it difficult to access CI and eventually showing high emission in solution. This difference mainly comes from the distinct electronic nature of two series; the negative charge of the cyano‐group dramatically stabilizes the FC structure of β‐series due to their bigger resonance structures, but in α‐series, this effect is relatively weaker. In the crystal state, every compound has a bright fluorescence because the significant intermolecular interaction prevented the rotation of a double bond.
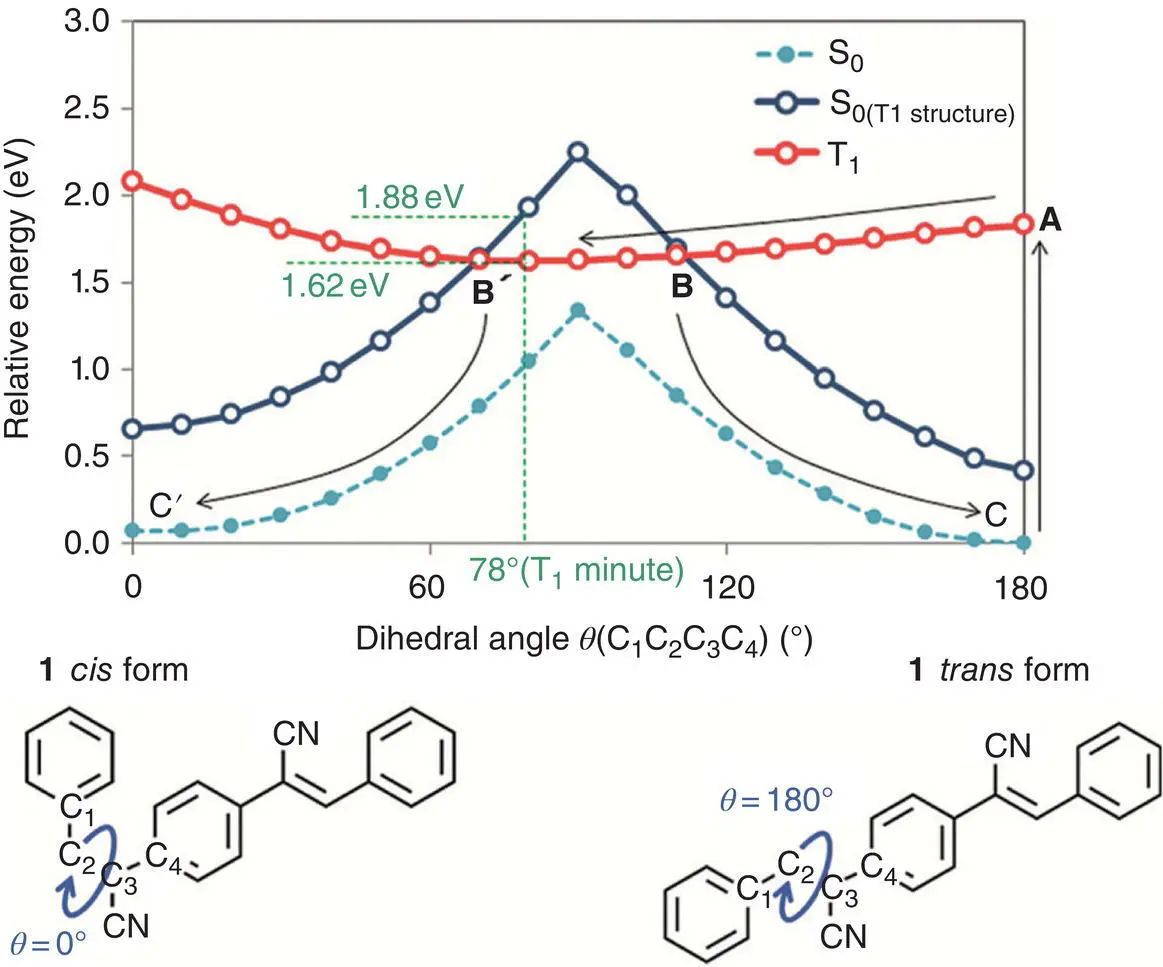
Figure 3.36 Potential energy curves of S 0at its optimized structures.
Source: Reproduced with permission from Ref. [62]. Copyright 2015, Wiley‐VCH Verlag GmbH &Co. KGaA.
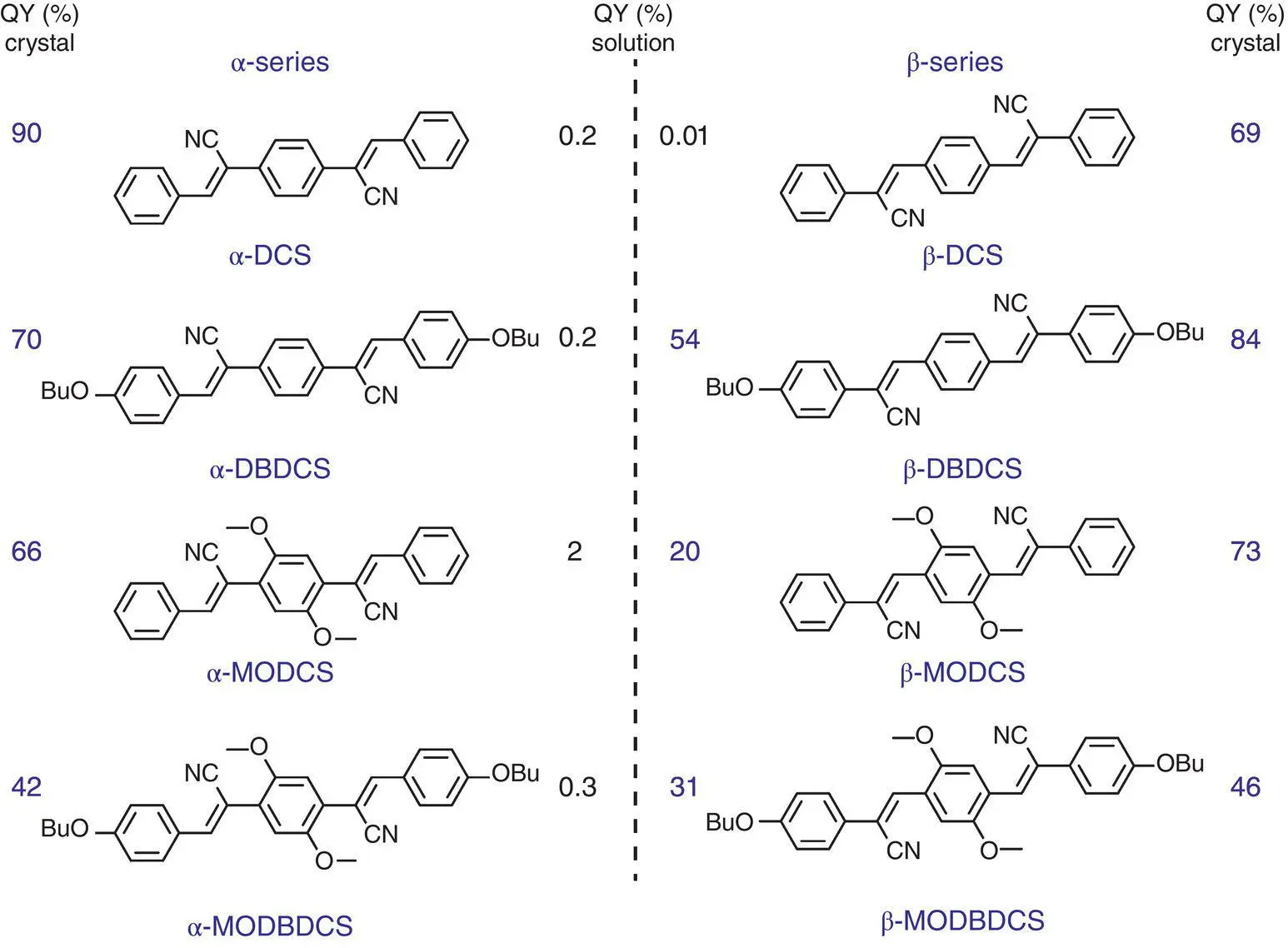
Figure 3.37 Molecular structure of α‐ (left) and β‐series (right) and their fluorescence quantum yields in CHCl 3and crystal [63].
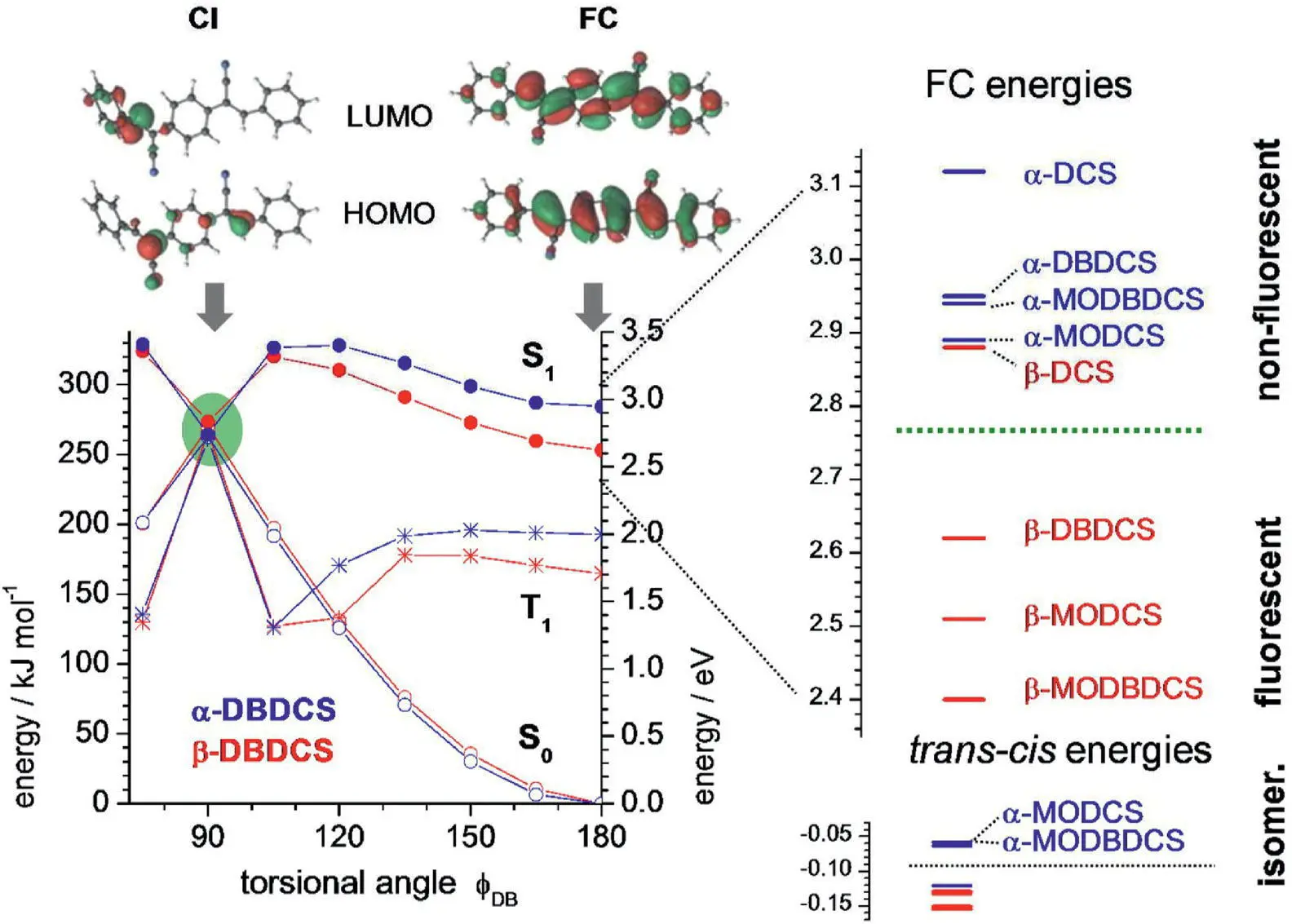
Figure 3.38 Left: TD‐DFT rigid torsional scans of one double bond φ DBfor α‐DBDCS and β‐DBDCS using the optimized S 0state in CHCl 3. Top: CASSCF calculated HOMO‐ and LUMO‐like orbitals. Right: TD‐DFT‐calculated FC energies and DFT‐calculated ground‐state energies.
Source: Reproduced with permission from Ref. [63]. Copyright 2017 American Chemical Society.
Diphenyl dibenzofulvene (DPDBF) is an AIEgen similar to TPE that was first reported by Tang et al. in 2007 [64]. Probably, the rotation of a double bond in DPDBF is responsible for fluorescence quenching of its solution similar to that of TPE. Shuai et al. [65] carried out a nonadiabatic dynamics simulation for the excited‐state nonradiative decay processes in open‐ and closed‐DPDBF and showed that the former exhibits an AIE property in contrast to the normal ACQ effect of the latter. The trajectory for open‐DPDBF showed that, after the initial excitation, the double bond length of open‐DPDBF increased quickly from its initial value of 1.37 to 1.55 Å after 10 fs, and the double bond rotation began correspondently (see Figure 3.39). There is a nonradiative transition point at 1206 fs; the energy for the S 0and S 1states approached each other with a gap of less than 0.5 eV. At this point, the two phenyl rings are nearly coplanar and the DBF ring is approximately perpendicular to the two phenyl rings. In contrast to open‐DPDBF, the C═C bond of closed‐DPDBF was restricted and the energy gap was relatively large at ~2 eV. Therefore, the energies of S 1could not release to S 0through such a point and emission was observed in solution.
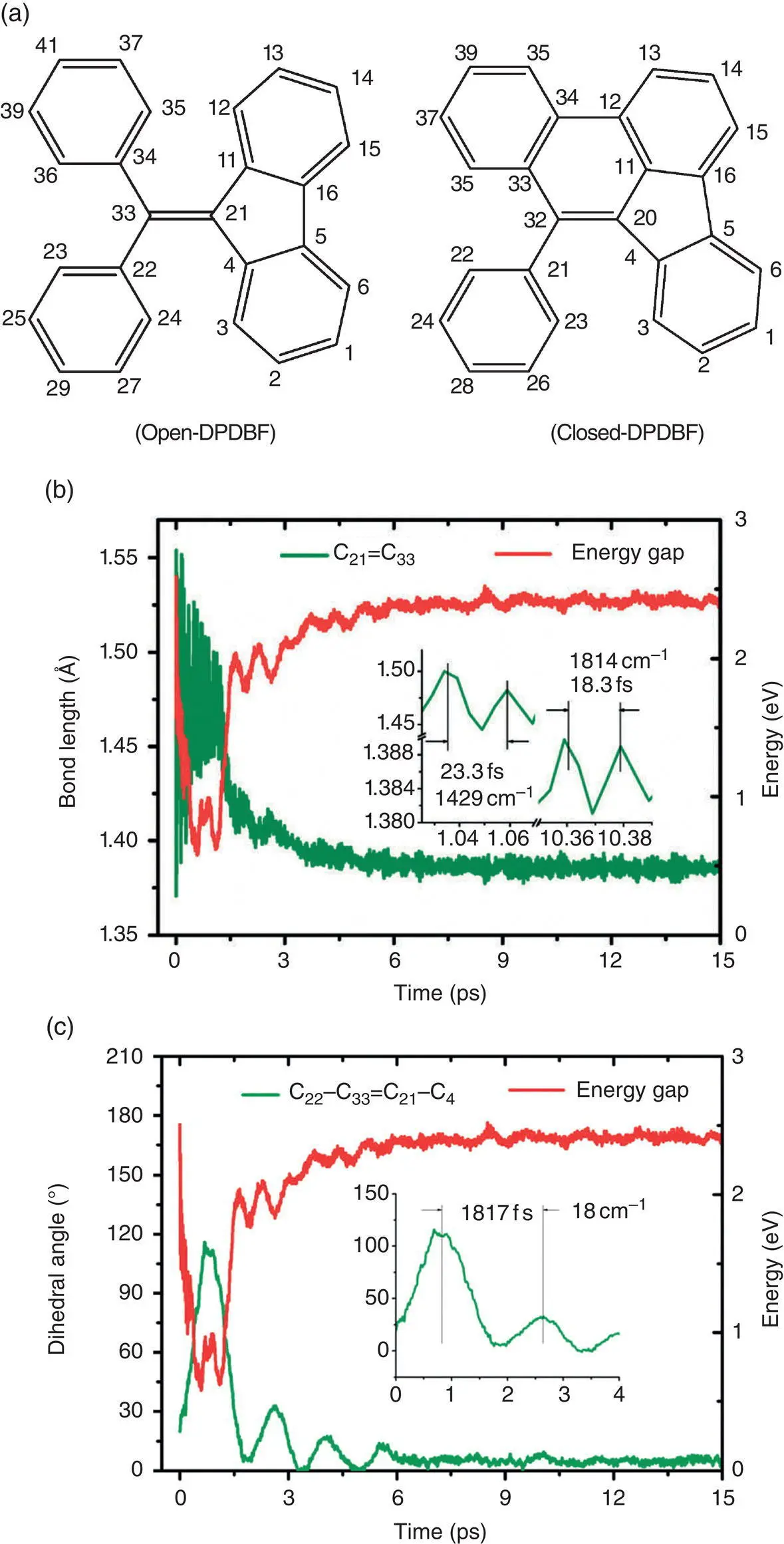
Figure 3.39 (a) The chemical structures for open‐ and closed‐DPDBF. The temporal evolution of the energy gap (S 1–S 0) (red) and the average values of coordinates (b) C 21=C 33(green) and (c) C 22–C 33=C 21–C 4(green).
Source: Reproduced with permission from Ref. [65]. Copyright 2012, Royal Society of Chemistry.
The studies reported above indicated that the single‐molecular nonradiative decay process of DPDBF mainly resulted from the rotation of the C═C bond, but further supplemental theoretical research of the AIE effect of DPDBF in the solid phase is needed. Blancafort et al. [66] combined solution and crystal computational simulation of DPDBF. In solution, the rotation of the C═C bond could reduce the energy of S 1and eventually decayed further to the ground state through a (S 1–S 0) CI seam. But in crystal, the rotation was hindered by the surrounding molecules, which caused the CI structure to show higher energy than S 1. The CI seam is disfavored for solid DPDBF, and fluorescent intensity is significantly enhanced (see Figure 3.40). In 2015, they further investigated the MECI of DPDBF in the crystal state [67]. A cluster of 12 molecules (528 atoms) surrounding each other was relaxed during the MECI optimization, with one molecule being treated at the QM level. The results confirmed that the AIE effect of DPDBF was due to the packing of the molecules. Even when the molecules surrounding the excited molecule were allowed to relax, the rotation of the C═C bond was still hindered and the CI responsible for nonradiative decay in solution is not accessible energetically.

Figure 3.40 Calculated mechanisms for the photophysics of DPDBF in acetonitrile (a) and in the solid phase (b).
Читать дальше

















