Source: Reproduced with permission from Ref. [66]. Copyright 2013, Royal Society of Chemistry.
In addition to these common AIE compounds discussed above, there are more examples to illustrate the importance of restricting the double bond rotation for certain AIEgens to render strong fluorescence. Liu et al. [68] report a computational study on the fluorescence quenching in methanol solution and fluorescence enhancement in crystal for 4‐diethylamino‐2 benzylidene malonic acid dimethyl ester (BIM).
The push−pull substitution of BIM could lead to a charge‐transfer (CT) structure and result in the fluorescence quenching of solution. The optimized results of the BIM molecule demonstrated that the double bond of the S 1state was greatly stretched and its torsion was more serious than S 0, but the twisting of single bonds in the vicinity of a double bond was reduced. An S 1minimum (referred to as S 1‐EM; see Figure 3.41) rendered weak emission and was energetically more stable than FC because of the torsion of a double bond. In addition, the rotation of both double bond and adjacent single bond could lead to the S 1state geometry relaxing to the CT structure without barriers. But the energetic decrease from the S 1state was much steeper for the former, suggesting that the former was the dominant S 1decay channel. Due to the excited molecules decaying to a CT state, the fluorescence of solution was quenched through the S 1/S 0CI near the CT intermediate.
In the crystal state, the simulation works revealed that the energetic difference between FC and S 1‐EM state was much slighter than that of BIM in solution, suggesting that the surrounding molecules restricted the rotation of both double bond and single bond and blocked the energetic relaxation from the intramolecular motions. Moreover, the energy of the CT state was higher than that of the FC state, and the energy barrier made it impossible for BIM nonradiative decay through forming CT intermediate. Consequently, high emission channel was accessible for BIM molecules in crystal states.
Tang et al. [69] prepared a series of benzylidene methyloxazolone (BMO) derivatives with AIE activities. EZI process was observed in one BMO derivative BMO‐PH by 1H NMR spectra (see Figure 3.42). When the Z ‐isomer in CDCl 3was irradiated by a UV light at 365 nm, the fraction of E ‐isomer increased quickly in the first 35 minutes. To investigate the relationship between the rotation of a double bond and solution fluorescence quenching, theoretical calculations were carried out. The theoretical calculations of BMO‐PH via DFT/TD‐DFT showed that in the ground state, the energy barrier of a double bond rotation was at least 1.0 eV higher than the single bond rotation. But in the S 1state, the barrier for the former was dramatically reduced and even lower than that for the latter. When torsion angles of the double bond were in the range of 70–120°, the formation of CI of S 1/S 0was mainly responsible for the nonradiative decay of BMO‐PH in the solution. In the crystal state, no EZI product was detected through 1H NMR, and high emission was observed.
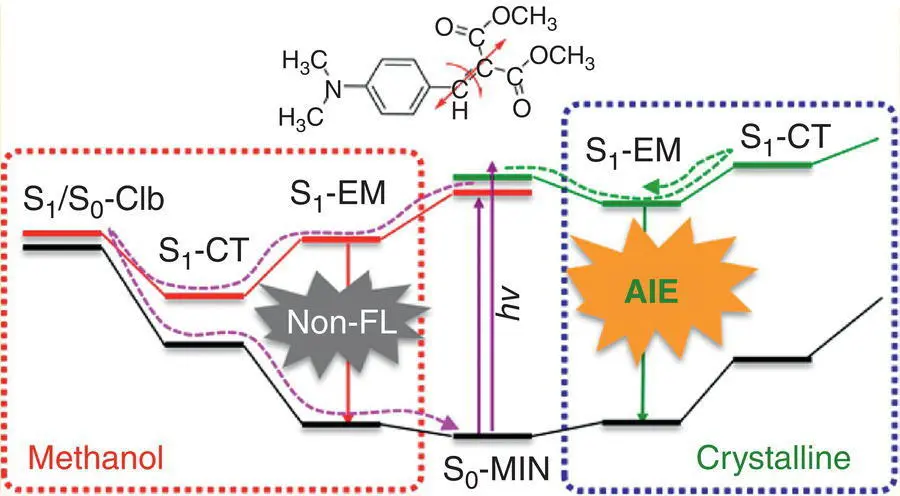
Figure 3.41 Schematic representation of the conical intersection (left) and AIE mechanisms in BIM.
Source: Reproduced with permission from Ref. [68]. Copyright 2016, American Chemical Society.
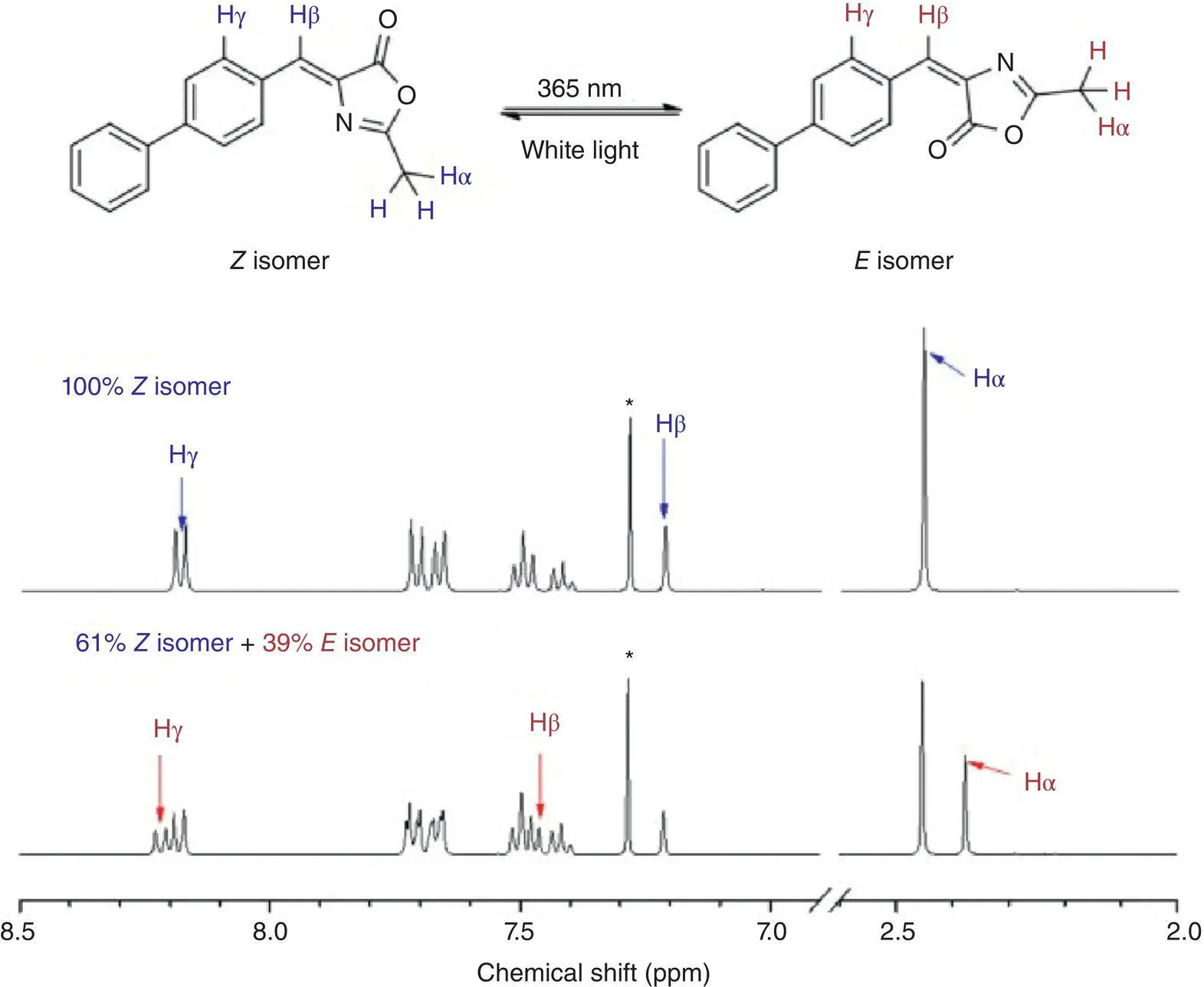
Figure 3.42 EZI process of BMO‐PH that was monitored by 1H NMR spectra. No irradiation (upper spectra) and irradiation (lower spectra) by a 365‐nm UV lamp for 35 minutes in CDCl 3(40 mM).
Source: Reproduced with permission from Ref. [69]. Copyright 2013, Royal Society of Chemistry.
Most of the AIE molecules, especially the most extensively studied TPE and its derivatives, possess a critical carbon–carbon double bond. Therefore, whether the RDBR process is involved and plays a key role in the AIE mechanism is a very concerned question even from the moment when the AIE phenomenon is discovered. From no effect, minor effect, to key effect and major effect on the AIE phenomenon, a long time has been passed. Now, the importance of the RDBR process in the AIE mechanism has been widely recognized and accepted. But the detailed relationship of the RDBR process and other nonradiative processes needs to be further unraveled and confirmed. The status and degree of the RDBR process, in general, RIR AIE mechanism, also needs to be further determined. More importantly, how the RDBR mechanism can be used to design better AIEgens is what we will do in the future. It is believed that more AIEgens that are based on RDBR mechanism and have exceptional properties will be developed in the near future.
1 1 Birks, J. B. (1970). Photophysics of Aromatic Molecules. London: Wiley.
2 2 Mei, J., Hong, Y., Lam, J. W. et al. (2014). Aggregation‐induced emission: the whole is more brilliant than the parts. Advanced Materials 26 (31): 5429–5479.
3 3 Lim, M. H. and Lippard, S. J. (2007). Metal‐based turn‐on fluorescent probes for sensing nitric oxide. Accounts of Chemical Research 40 (1): 41–51.
4 4 Tang, C. W. and Vanslyke, S. A. (1987). Organic electroluminescent diodes. Applied Physics Letters 51 (12): 913–915.
5 5 Luo, J., Xie, Z., Lam, J. W. Y. et al. (2001). Aggregation‐induced emission of 1‐methyl‐1,2,3,4,5‐pentaphenylsilole. Chemical Communications 381 (18): 1740–1741.
6 6 Hu, R., Lam, J. W. Y., Liu, Y. et al. (2013). Aggregation‐induced emission of tetraphenylethene‐hexaphenylbenzene adducts: effects of twisting amplitude and steric hindrance on light emission of nonplanar fluorogens. Chemistry A European Journal 19 (18): 5617–5624.
7 7 Tong, H., Hong, Y., Dong, Y. et al. (2006). Fluorescent “light‐up” bioprobes based on tetraphenylethylene derivatives with aggregation‐induced emission characteristics. Chemical Communications ( 35): 3705–3707.
8 8 An, B.‐K., Kwon, S.‐K., Jung, S.‐D. et al. (2002). Enhanced emission and its switching in fluorescent organic nanoparticles. Journal of the American Chemical Society 124 (48): 14410–14415.
9 9 Kokado, K. and Chujo, Y. (2009). Polytriazoles with aggregation‐induced emission characteristics: synthesis by click polymerization and application as explosive chemosensors. Macromolecules 42 (5): 1421–1424.
10 10 Wang, M., Zhang, G., Zhang, D. et al. (2010). Fluorescent bio/chemosensors based on silole and tetraphenylethene luminogens with aggregation‐induced emission feature. Journal of Materials Chemistry 20 (10): 1858–1867.
11 11 Chen, J., Law, C. C. W., Lam, J. W. Y. et al. (2003). Synthesis, light emission, nanoaggregation, and restricted intramolecular rotation of 1,1‐substituted 2,3,4,5‐tetraphenylsiloles. Chemistry of Materials 15 (7): 1535–1546.
12 12 Mei, J., Leung, N. L. C., Kwok, R. T. K. et al. (2015). Aggregation‐induced emission: together we shine, united we soar! Chemical Reviews 115 (21):11718–11940.
13 13 Feng, H.‐T., Yuan, Y.‐X., Xiong, J.‐B. et al. (2018). Macrocycles and cages based on tetraphenylethylene with aggregation‐induced emission effect. Chemical Society Reviews 47 (19): 7452–7476.
14 14 Hong, Y., Lam, J. W. Y., and Tang, B. Z. (2011). Aggregation‐induced emission. Chemical Society Reviews 40 (11): 5361−5388.
15 15 Kwok, R. T. K., Leung, C. W. T., Lam, J. W. Y. et al. (2015). Biosensing by luminogens with aggregation‐induced emission characteristics. Chemical Society Reviews 44 (33): 4228−4238.
Читать дальше














