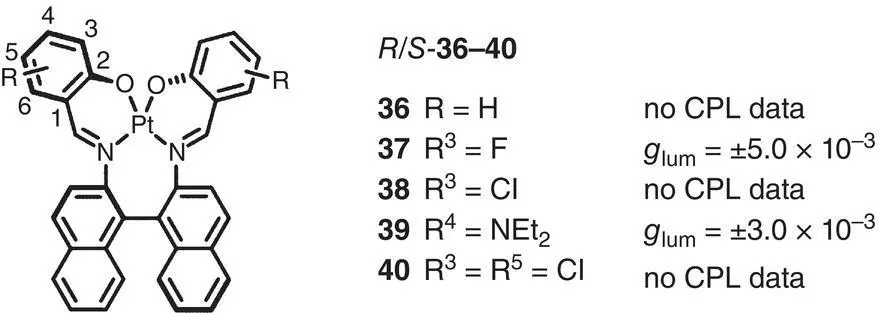
Figure 2.9 Molecular structures of chiral Pt(II)‐Salen complexes 36– 40and corresponding g lum[29].
Recently, several AICPL systems based on chiral clusters were also investigated. In 2017, Tang and coworkers synthesized a chiral Au 3cluster with unique optical absorption activity ( Figure 2.10) [31]. For an individual cluster 41, nearly no luminescence was observed. Interestingly, due to the strong CH/ π interaction, 41self‐organized into nanocubes with a body‐centered cubic packing in a CH 2Cl 2/hexane mixture and exhibited intense CPL signal with g lumof ±7.0 × 10 −3(583 nm).
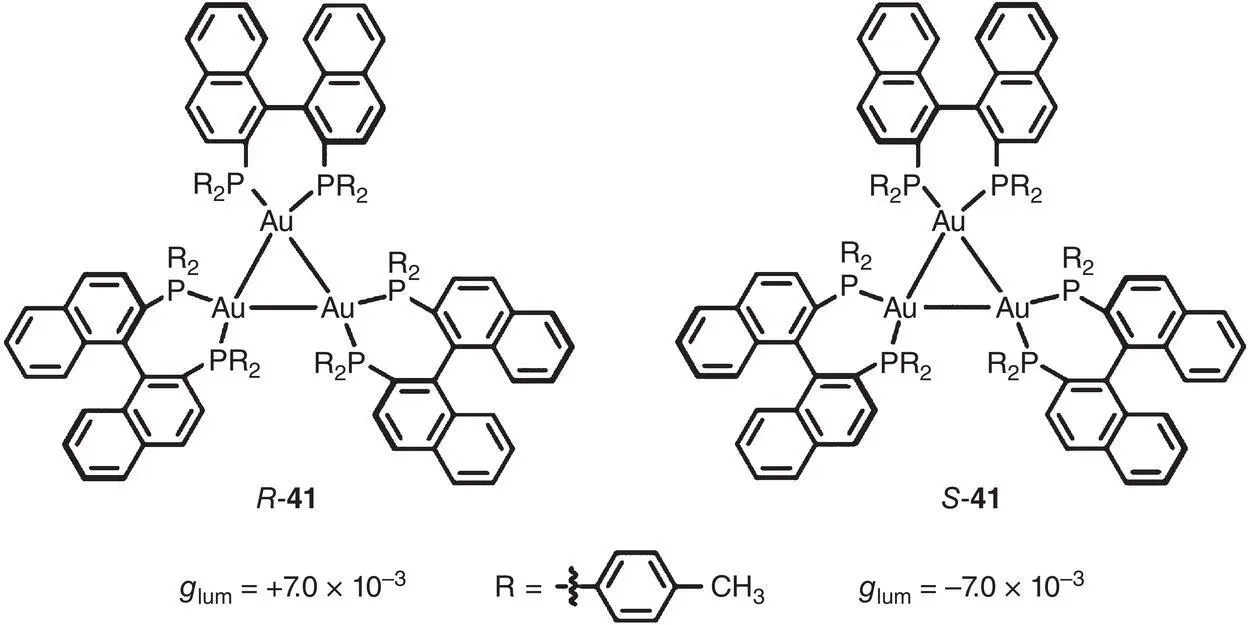
Figure 2.10 Molecular structures of chiral Au 3cluster enantiomers R ‐ 41and S ‐ 41and corresponding g lum[31].
In 2019, Zang and coworkers prepared an atomically precise chiral copper(I) cluster 42([Cu 14( R / S ‐DPM) 8](PF 6) 6) with a typical AIE feature and CPL activity from chiral ligands R ‐DPM and S ‐DPM ( Figure 2.11) [32]. The PL intensity of 42increased when the fraction of n ‐hexane increased in the CH 2Cl 2/hexane mixtures. In a dimethyl sulfoxide (DMSO)/H 2O system, similar AIE effect was also observed. As for chiroptical properties, obvious CPL signals were observed with g lumup to ±1.0 × 10 −2(702 nm) for R ‐ 42and S ‐ 42when the fraction of n ‐hexane exceeded 40%.
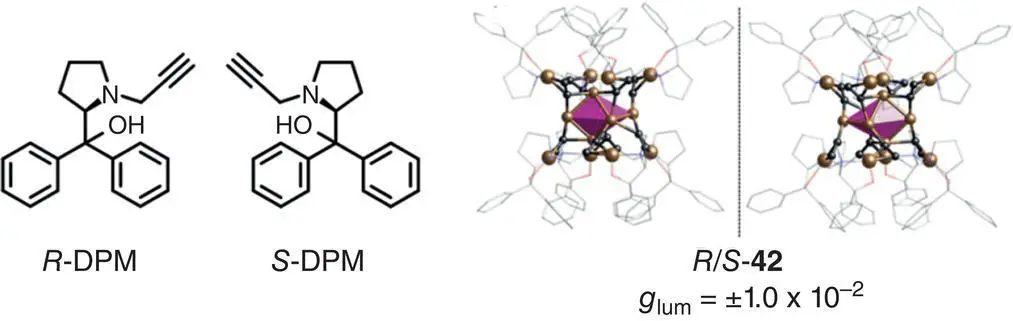
Figure 2.11 (a) Molecular structures of chiral ligands R ‐DPM and S ‐DPM. (b) Single crystal structures of R ‐ 42and S ‐ 42, and corresponding g lumin CH 2Cl 2/hexane mixtures.
Source: Reproduced with permission [32]. Copyright 2019, Wiley‐VCH.
In 2020, a propeller‐like chiral trinuclear copper(I) cluster 43was reported by Zang’s group ( Figure 2.12) [33]. By introducing several flexible phenyl groups into the cluster, 43was endowed with AIE activity following the RIM mechanism. CD spectra indicated that the chirality belonged to cluster 43rather than the isolated chiral ligands. In the aggregated state (in DMSO/H 2O mixtures), remarkable CPL signal was observed with g lumof ±2.0 × 10 −2(610 nm).
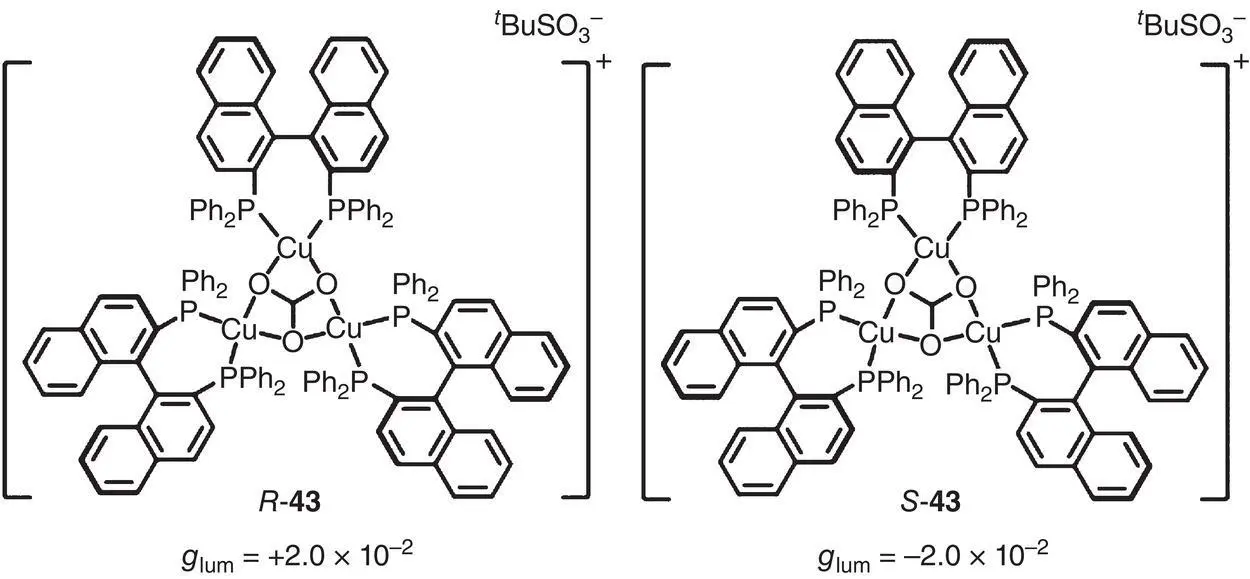
Figure 2.12 Molecular structures of chiral copper cluster enantiomers R ‐ 43and S ‐ 43and corresponding g lum[33].
2.5 Supramolecular Systems
Recently, supramolecular self‐assembly of chiral AIEgens guided by relatively weak interactions is emerging as an efficient way to prepare CPL‐active materials. In 2012, Liu et al. introduced two mannose‐containing side chains into a tetraphenylsilole derivative and prepared a novel chiral AIEgen 44( Figure 2.13) [34]. Further experiments demonstrated that the CPL performance of 44was highly dependent on the self‐assembly conditions. Without a control over the self‐assembly morphology, no obvious CPL signals could be observed. On the contrary, the highly ordered self‐assembly structure formed in the microfluidic channels generated strong CPL with g lumup to −0.32 (451 nm). This work pioneered the design of AICPL materials via supramolecular self‐assembly.
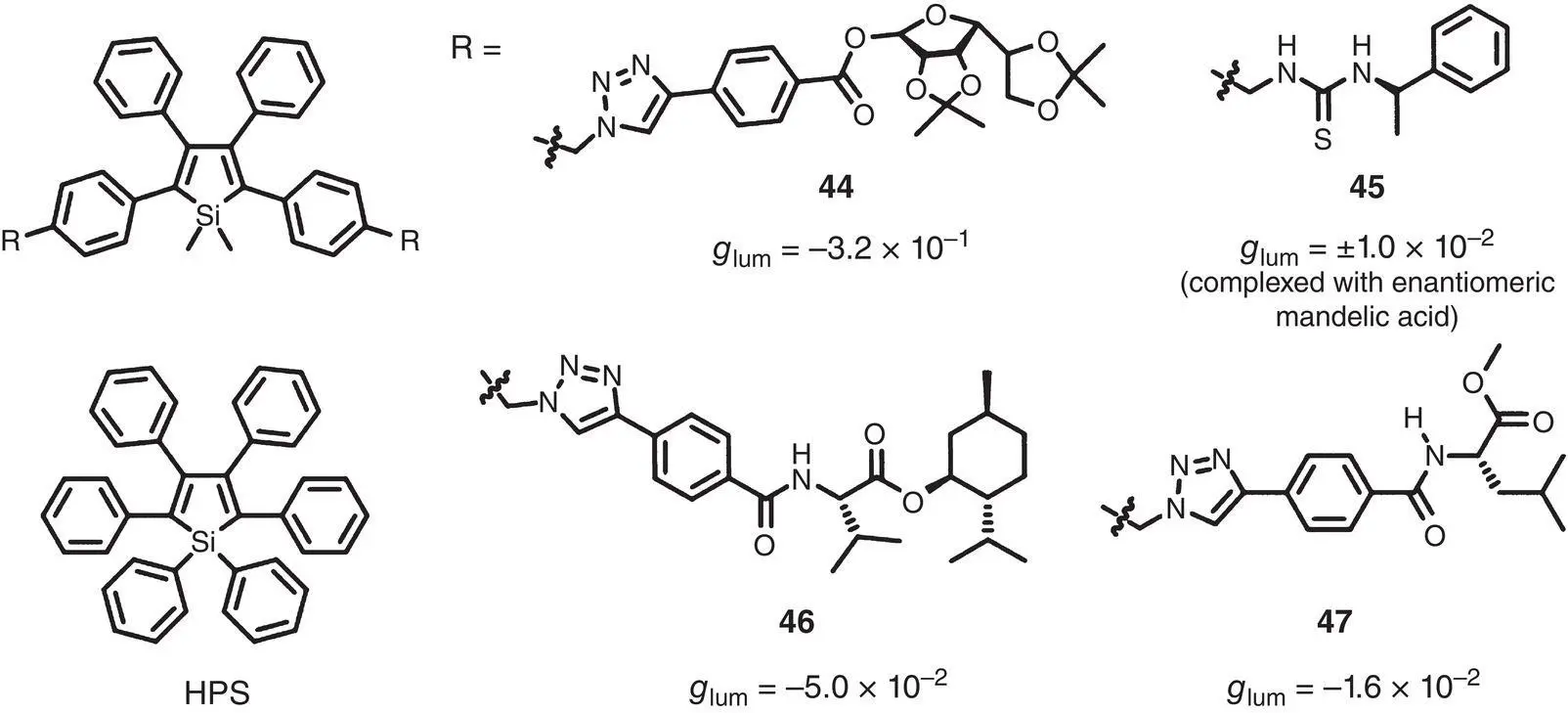
Figure 2.13 Molecular structures of chiral silole‐based AIEgens 44– 47and corresponding g lum[34–37].
Coming down in one continuous line, several chiral AIEgens 45– 47based on silole derivatives were reported by Tang’s group ( Figure 2.13) [35–37]. Compound 45was comprised of a tetraphenylsilole luminescent core and several chiral phenylethanamine pendants and showed a typical AIE feature [35]. It was CD and CPL‐silent in solution or in a film probably due to the low efficiency of chirality transcription from the chiral side chains to the tetraphenylsilole core. However, after being mixed with chiral acids, such as R ‐ or S ‐mandelic acid, 45revealed intense CPL signals centered around 500 nm with high | g lum| of 0.01. This was attributed to the formation of ordered supramolecular structures. Compounds 46and 47were synthesized by combining an AIE‐active silole unit and various chiral pendants (valine‐ or leucine‐containing side chains) via click chemistry reactions. Compound 46self‐assembled into helical structures after drying from a THF solution or from the THF/H 2O mixtures and exhibited strong CPL with high g lumof −5.0 × 10 −2(500 nm) [36]. For compound 47, similar helical self‐assembles were observed after drying from a CH 2Cl 2solution and the micropatterned structure formed in the microfluidic channels showed CPL with g lumof −1.6 × 10 −2(416 nm) [37]. In 2017, Tang’s group found that the unmodified HPS exhibited CPL with g lumas high as −1.25 × 10 −2(550 nm) in a crystalline film due to the formation of well‐ordered helical self‐assembly [38].
As another commonly used AIEgen, TPE was also used to design novel chiral AIEgens. Monofunctionalized TPE‐based chiral AIEgens 48and 49and difunctionalized chiral AIEgens 50and 51have been reported by Tang’s group since 2014 ( Figure 2.14) [39–42]. The TPE units were modified with valine‐ or leucine‐derived side chains via click chemistry reactions. With the help of the chiral side chains, compounds 48– 51formed helical aggregates. On the other hand, the AIE‐active TPE units endowed these chiral self‐assembles with strong blue luminescence as well as intense CPL. For CPL performance, the monofunctionalized chiral AIEgens exhibited CPL with high g lumof +3 × 10 −2(445 nm) and +5 × 10 −2(450 nm) for 48and 49, respectively. However, the difunctionalized TPE‐based chiral AIEgens 50and 51exhibited CPL with relatively lower | g lum| of 3.2–5.3 × 10 −3(430–440 nm).
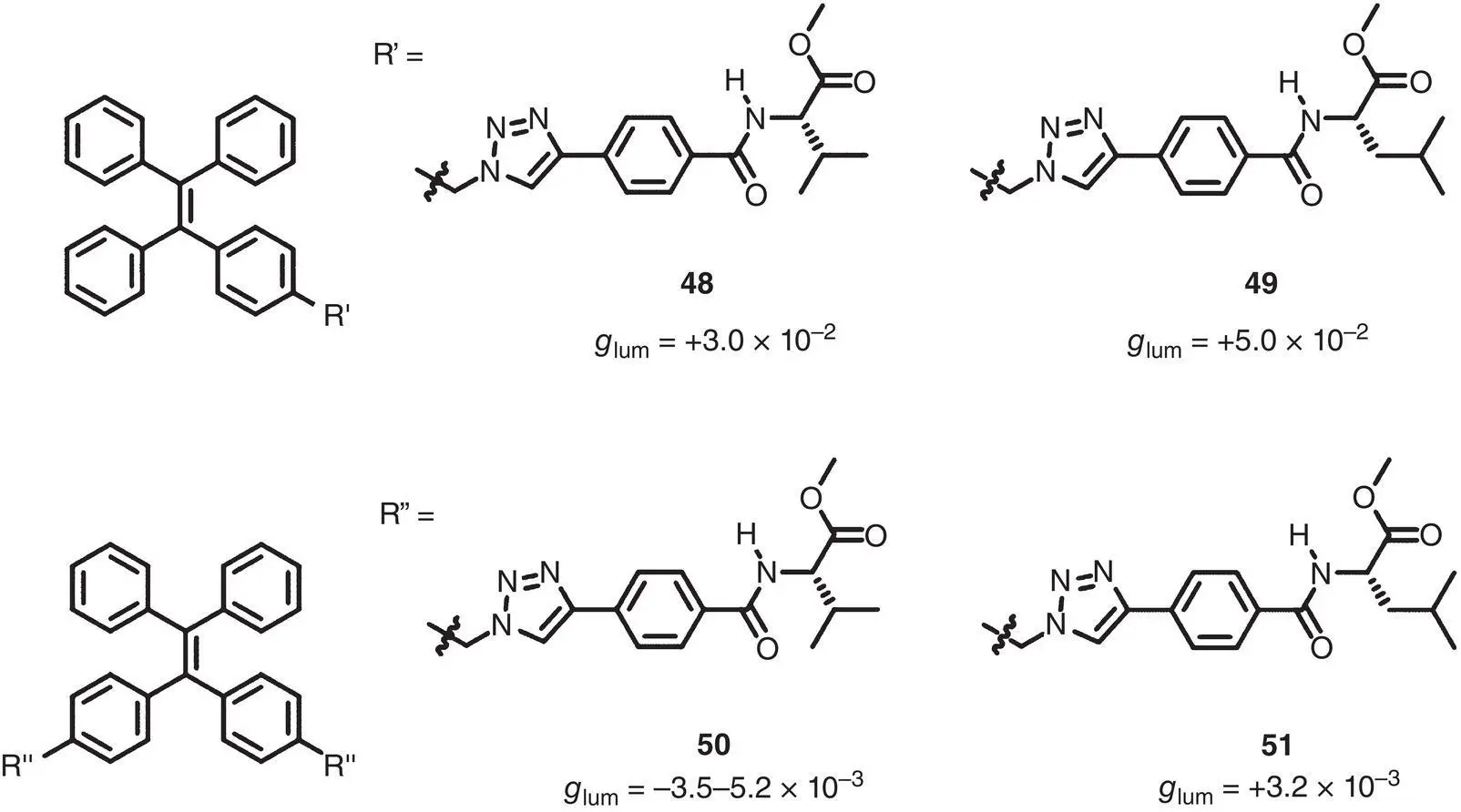
Figure 2.14 Molecular structures of chiral TPE‐based AIEgens 48– 51and corresponding g lum[39–42].
Читать дальше


















