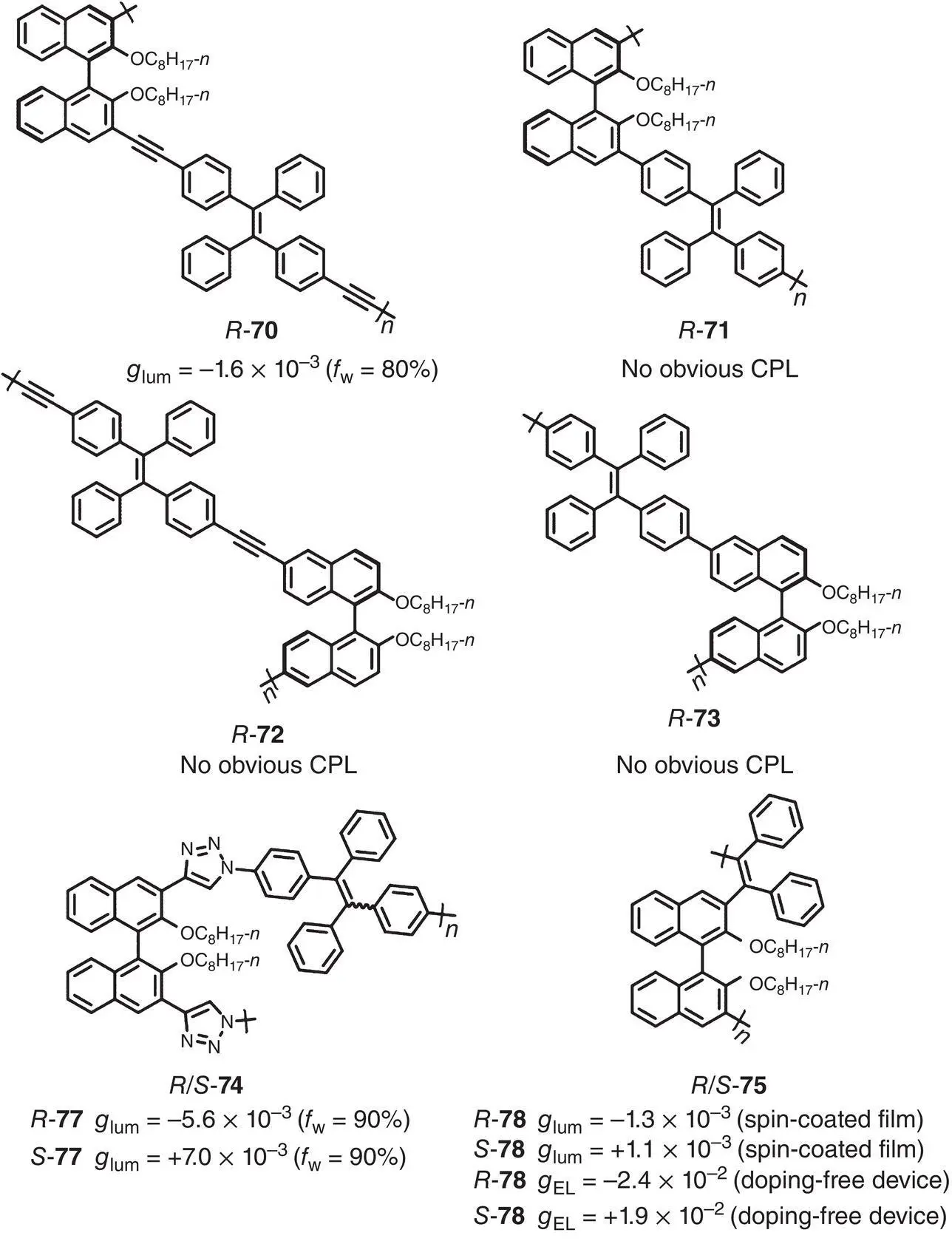
Figure 2.25 Molecular structures of AIE‐active chiral conjugated polymers 70– 75and corresponding g lum[58–60].
In 2017, Cheng et al. reported five three‐component chiral conjugated polymers 76– 80incorporating the binaphthyl, fluorine, and TPE moieties, which functioned as the chiral source, Förster resonance energy transfer (FRET) donor and FRET acceptor, respectively ( Figure 2.26) [61]. The polymers showed CPL with | g lum| of 2.0–4.0 × 10 −3(480 nm). In the same year, Cheng et al. prepared two chiral conjugated polymers 81and 82, which were comprised of four components as well as two FRET pairs ( Figure 2.26) [62]. The fluorene moiety and the TPE unit formed the first FRET pair, and the later formed the second FRET pair with 4,7‐di(thiophen‐2‐yl)‐2,1,3‐benzothiadiazole moiety. Due to the existence of two FRET pairs, deep‐red luminescence with large Stokes shifts was observed. In addition, the g lumreached up to ±2.0 × 10 −3(around 650 nm) in the aggregated state ( f w= 99%).
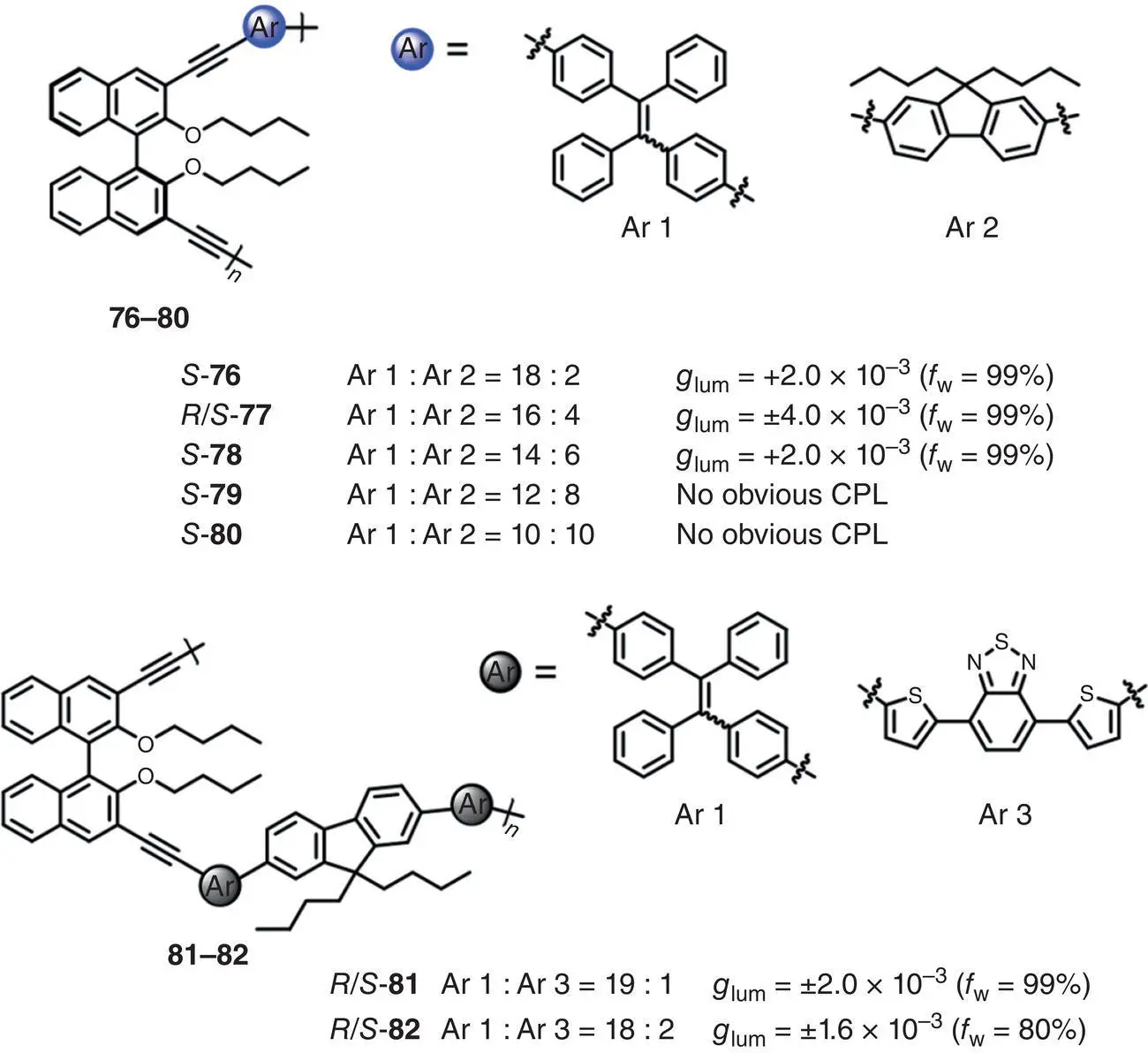
Figure 2.26 Molecular structures of AIE‐active three‐component chiral conjugated polymers 76– 80and AIE‐active four‐component chiral conjugated polymers 81– 82and the corresponding g lum[61, 62].
In 2018, Chen’s group synthesized a pair of conjugated polymers P ‐ 83and M ‐ 83with the TPE units and the chiral helical aromatic esters immobilized in the main chain ( Figure 2.27) [63]. According to PL spectra, polymer 83possessed a typical AIE feature with enhanced luminescence in the aggregated state. In THF solution, polymer 83was CPL‐inactive. Upon mixing with water, AICPL centered at 500 nm was observed with g lumof ±8.3 × 10 −4.
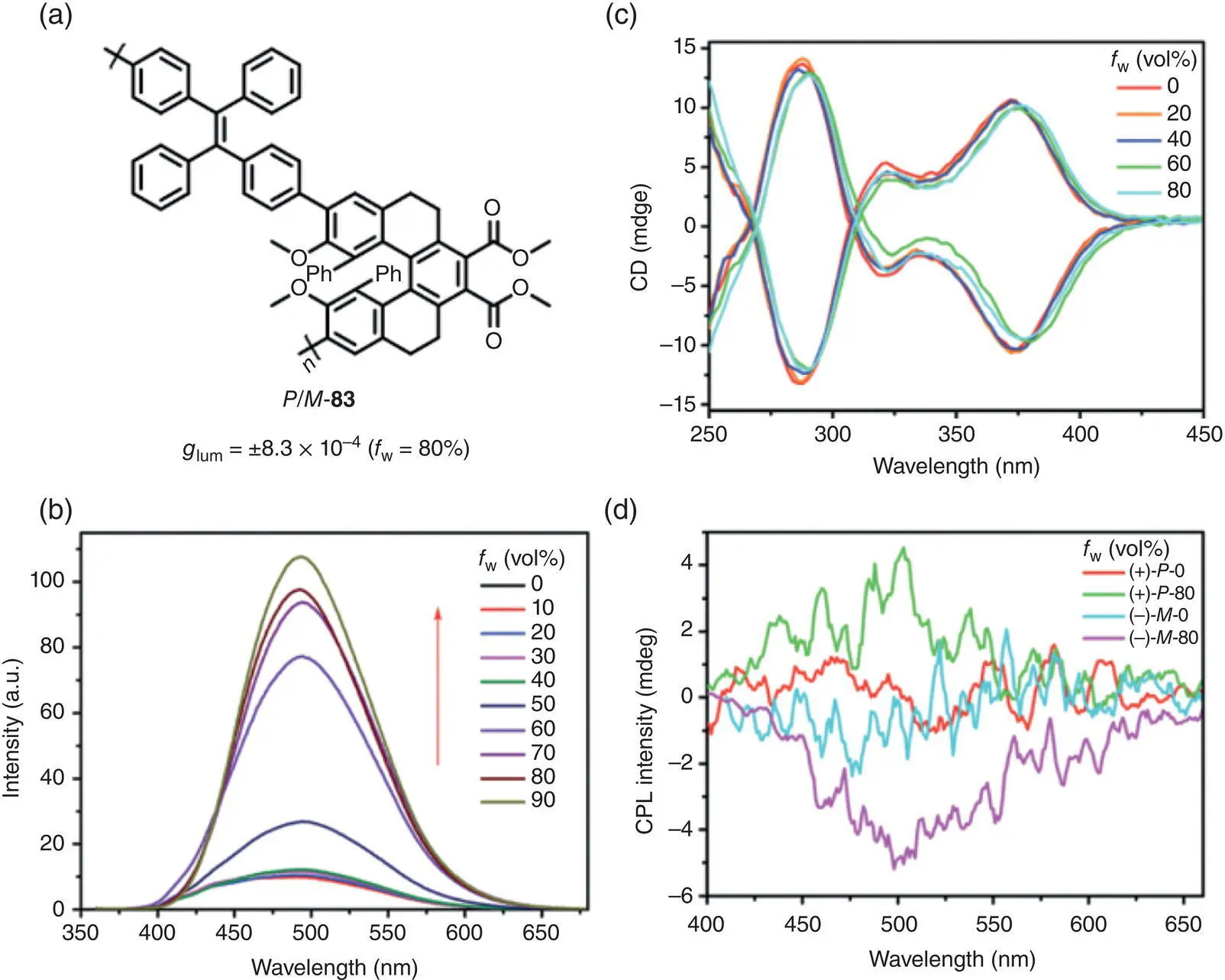
Figure 2.27 (a) Molecular structures of AIE‐active chiral conjugated polymers P ‐ 83and M ‐ 83and corresponding g lum. (b) PL, (c) CD spectra, and (d) CPL spectra of polymer 83in various THF/H 2O mixtures.
Source: Reproduced with permission [63]. Copyright 2018, The Royal Society of Chemistry.
In 2018, Tang’s group prepared an AIE‐active chiral polymer 84by connecting alanine‐containing pendants and TPE units together via Cu(I)‐catalyzed click polymerization ( Figure 2.28) [64]. In a dilute THF solution, the polymer exhibited weak CD and no apparent CPL signals. In the cast film, intense CPL around 500 nm was observed with g lumof +4.5 × 10 −3.
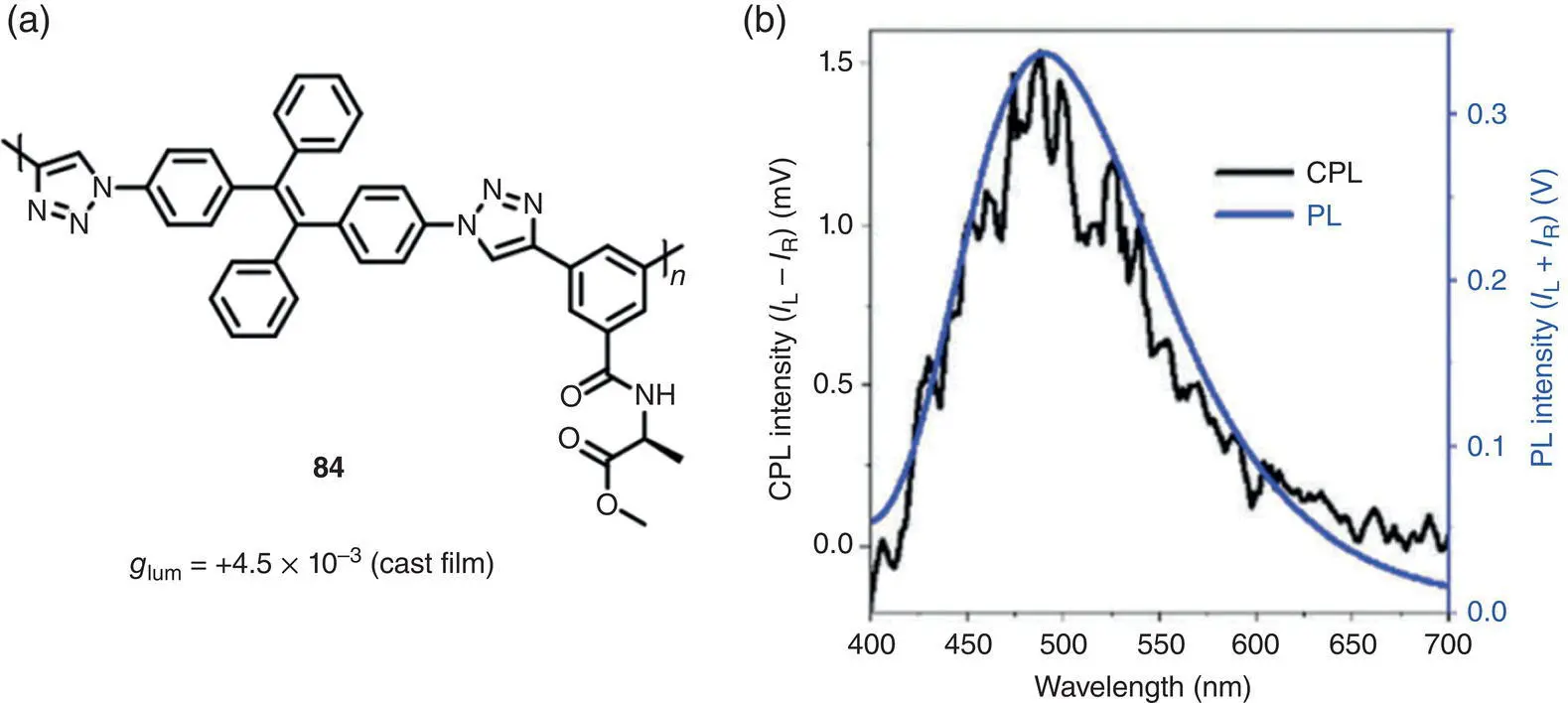
Figure 2.28 (a) Molecular structure of chiral conjugated polymer 84and corresponding g lum. (b) PL and CPL spectra of polymer 84in a cast film.
Source: Reproduced with permission [64]. Copyright 2018, The Royal Society of Chemistry.
CPL‐active liquid crystal (LC) systems based on AIEgens were also investigated. The helical orientation of the LC molecules appears to be critical for the CPL performance. Both helical stacking of chiral AIEgens and doping achiral AIEgens in chiral nematic LCs can generate CPL.
In 2015, Lu et al. prepared a novel AIE‐active chiral LC molecule 85by introducing two cholesterol pendants into a TPE unit with long alkyl chains ( Figure 2.29) [65]. With a flexible molecular structure, molecule 85formed a cholesteric LC and exhibited temperature‐dependent chiroptical properties. The resulting LC showed a prominent CPL performance with g lumof +2.5 × 10 −2(483 nm) at 20 °C, and declined to +7.5 × 10 −3(480 nm) at 40–50 °C. Above 60 °C, the mixture was almost CPL‐inactive. The temperature‐sensitive CPL was consistent with the trend of CD signals. The fact that the CD and CPL signals changed with temperature was presumably due to the variations of the helical pitch of the cholesteric phase. Polarizing optical microscopy images further proved the hypothesis. In 2019, Yang et al. prepared two LC molecules by modifying a TPE unit with four cholesteric‐derived side chains [66]. Through ordered columnar self‐assembly, these molecules formed a liquid crystalline mesophase and generate CPL with g lumup to +8.8 × 10 −2(491 nm).
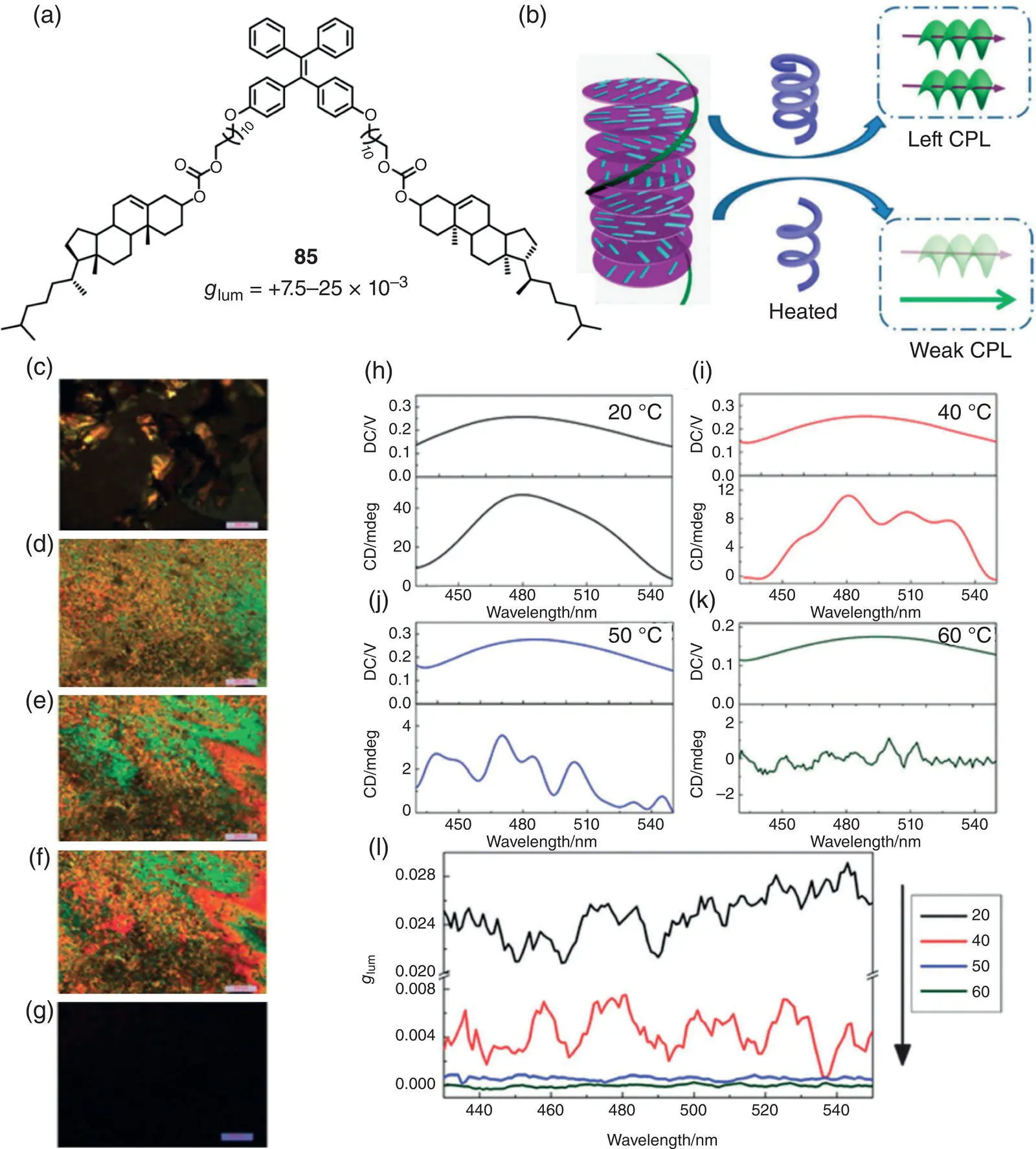
Figure 2.29 (a) Molecular structure of 85and corresponding g lum. (b) Schematic illustration of temperature‐dependent CPL. (c–g) Polarizing optical microscopy images of 85at different temperatures: (c) 20 °C, (d) 50 °C, (e) 60 °C, (f) 75 °C, and (g) 90 °C, scale bar: 200 μm. (h–k) CPL spectra of 85at different temperatures: (h) 20 °C, (i) 40 °C, (j) 50 °C, and (k) 60 °C. (l) g lumfactor vs. wavelength plot at different temperature.
Source: Reproduced with permission [65]. Copyright 2015, The Royal Society of Chemistry.
In 2016, Tang et al. reported a CPL‐active system with achiral TPE‐derived molecule 86( Figure 2.30) doped in a chiral nematic liquid crystal (N*LC) [67]. The resulting LC favored the fabrication of a display with | g lum| as high as 0.4.
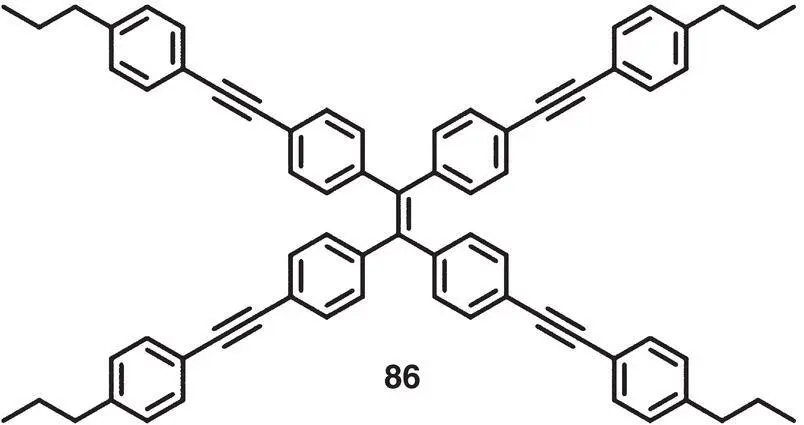
Figure 2.30 Molecular structure of achiral AIEgen 86[66].
In 2018, Cheng et al. prepared an achiral emissive nematic liquid crystal by doping BINOL‐derived AIEgen enantiomers R / S ‐ 87into a common achiral nematic liquid crystal (N‐LC E7) ( Figure 2.31) [68]. The products exhibited AICPL centered at 536 nm with high | g lum| up to 0.41. Later in 2019, the same group reported a subsequent work on a three‐component chiral nematic liquid crystal system ( Figure 2.32) [69]. As shown in Figure 2.32c, an achiral nematic liquid crystal was used as the host, and then doped with chiral BINOL‐derived dopant R / S ‐ 88and achiral AIEgens 89– 92. The resulting chiral nematic liquid crystals showed strong CPL with | g lum| in the range of 0.97–1.42 (505–610 nm). In 2019, Duan et al. also reported a doped chiral LC system with | g lum| up to 0.27 [70]. Recently, Cheng et al. prepared two D–A type chiral AIEgens and prepared chiral nematic liquid crystals with strong CPL around 550 nm with g lumof ±0.37 [71].
Читать дальше


















