In 2019, Zhang and Cheng et al. prepared four chiral AIEgens 52– 54combining a TPE unit and one or two chiral glutamic acid‐derived side chains ( Figure 2.15) [43]. The enantiomers of monosubstituted molecule 52exhibited CPL with g lumup to ±2.0 × 10 −2(450 nm). As for disubstituted molecules, CPL signals with g lumof −7.0 × 10 −3(500 nm) and −8.0 × 10 −3(500 nm) were observed for 53and 54, respectively.
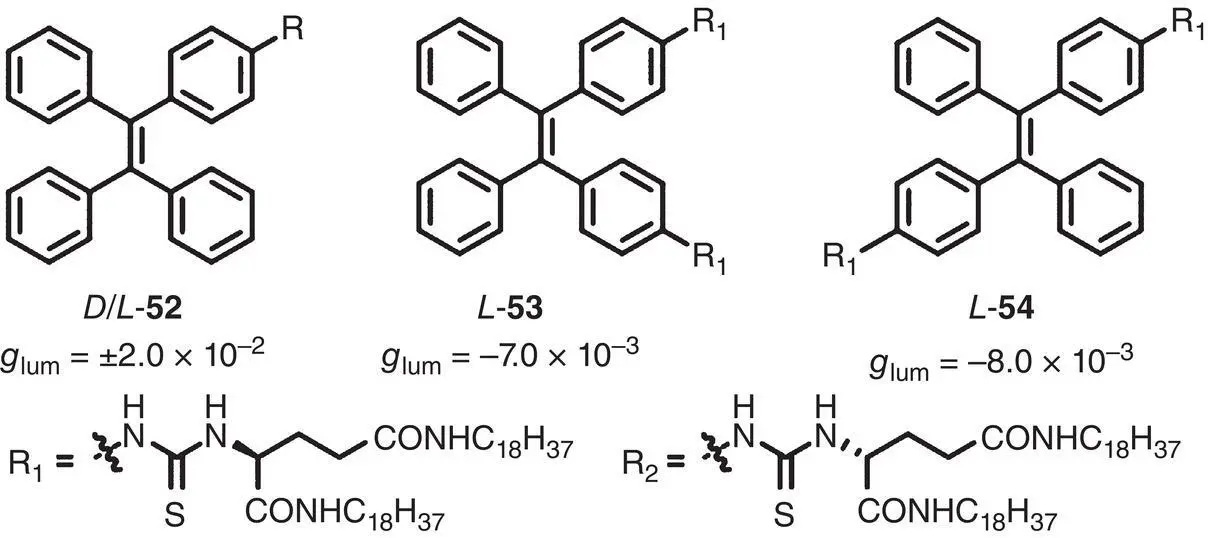
Figure 2.15 Molecular structures of chiral TPE‐based AIEgens 52– 54and corresponding g lum[43].
In 2018, Zheng’s group reported a TPE‐based triangular macrocycle 55, which was decorated with three crown ether rings ( Figure 2.16) [44]. The macrocycle was achiral itself, but exhibited CD and CPL signals after the addition of chiral acids and the | g lum| (520 nm) was between 1.0 × 10 −3and 2.1 × 10 −3. According to the proposed mechanism, the host–guest interaction between the crown ether rings and the chiral acids may render a single chirality of the TPE unit prevailing inside a macrocycle and hence lead to CD and CPL activities. Later in 2019, the same group prepared macrocycles 56and 57by connecting TPE dicycle or TPE unit with four chiral cholesterol groups [45]. CPL spectra showed that the dual cycle structure played an important role in single chirality induction. Thus, macrocycle 56with such a structure showed higher CPL activity (| g lum| = 3.0 × 10 −3at 450 nm) than macrocycle 57(| g lum| = 1.0 × 10 −4at 475 nm).
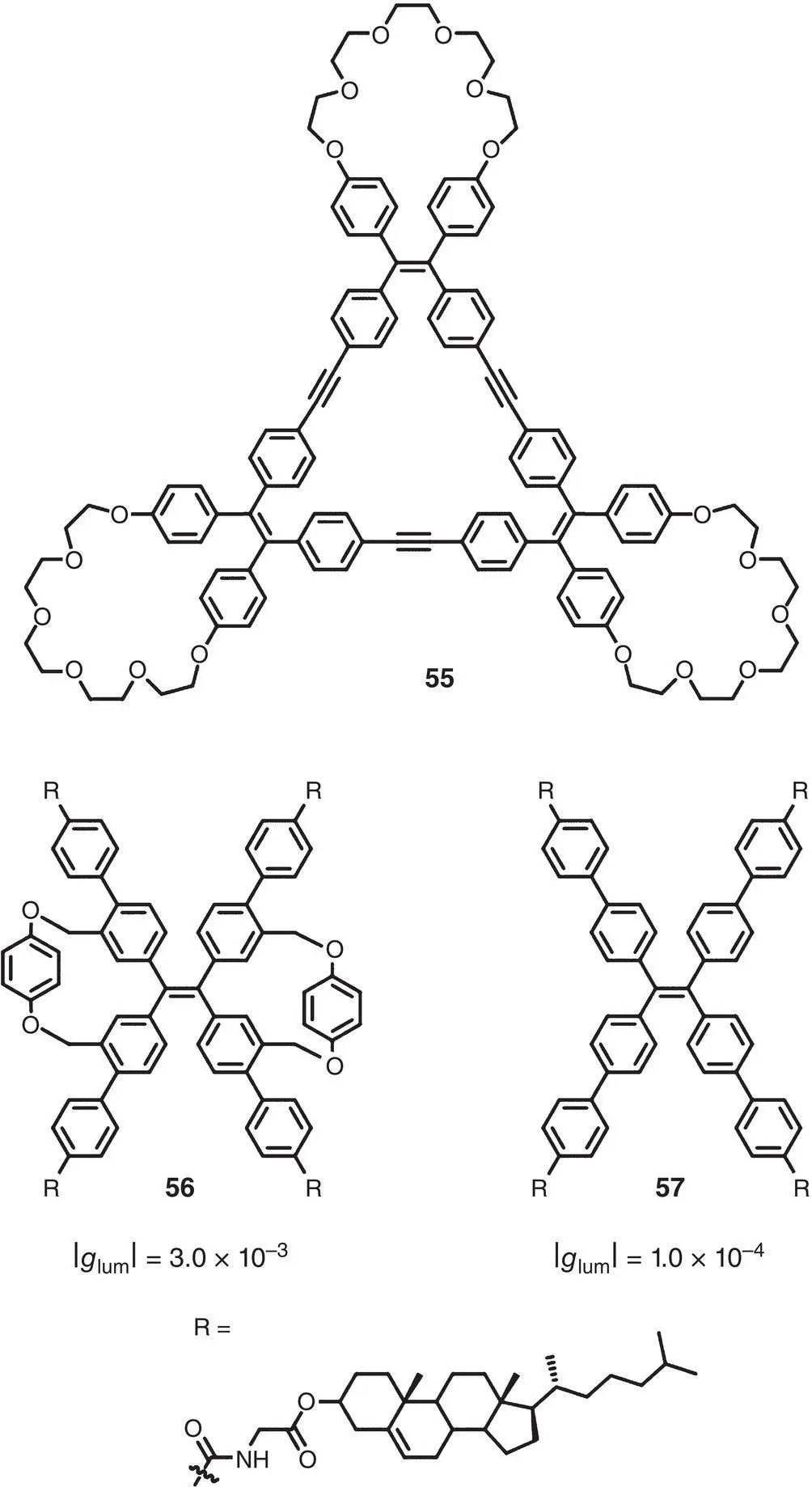
Figure 2.16 Molecular structures of triangular macrocycle 55, TPE dual cycle tetracholesterol 56and TPE tetracholesterol 57, and corresponding g lum[44, 45].
Besides the silole‐ and TPE‐based molecules, other chiral AIE‐active systems were also investigated in the supramolecular systems. In 2018, Huang et al. synthesized two chiral alanine‐containing Schiff base with AIE activities, which was self‐assembled into a helical structure and exhibited CPL with g lumup to +1.3 × 10 −2(650 nm) [46]. Recently, Jiang and coworkers developed a gel system based on a novel chiral AIEgen 58, which was synthesized by connecting pyridine functionalized cyanostilbene with a chiral cholesterol unit through an ester linker ( Figure 2.17) [47]. Due to the chirality transfer and amplification during the gelation process, 58exhibited intense blue CPL with high g lumof −3.0 × 10 −2(480 nm) and −1.7 × 10 −2(480 nm) for the gel and xerogel film, respectively. More interestingly, reversible luminescence modulation was realized by protonation and deprotonation of the pyridine group. The color was highly tunable between blue and orange (480–530 nm) by varying the exposure of trifluoroacetic acid, without any significant change of g lum.
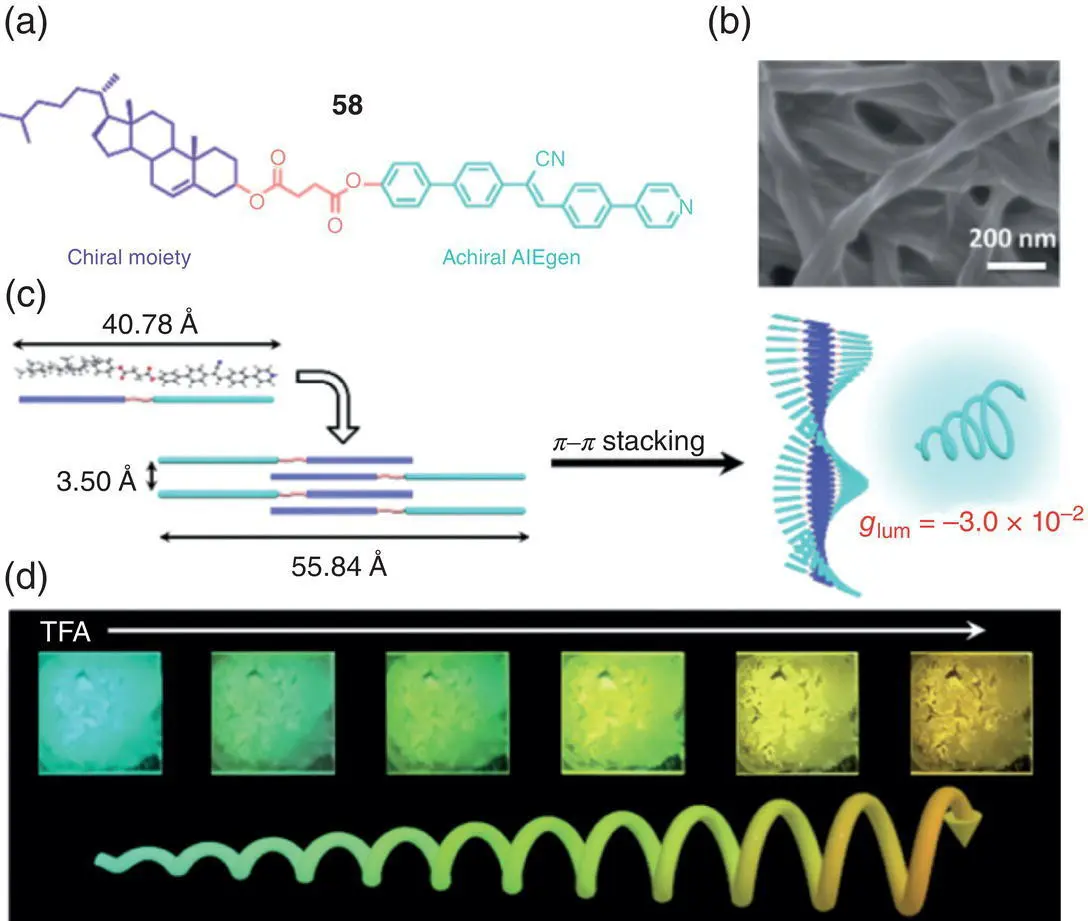
Figure 2.17 (a) Molecular structure of chiral AIEgen 58. (b) Scanning electron microscope image of xerogel dried from DMSO. (c) Proposed molecular packing of chiral AIEgen 58for blue CPL. (d) Multicolor CPL upon exposure of the xerogel films to different amount of TFA.
Source: Reproduced with permission [47]. Copyright 2020. Wiley‐VCH.
In contrast to the above‐mentioned pure organic CPL‐active systems, Ikeda et al. prepared a Pt(II) complex 59with chiral alkyl side chains, which revealed solvent‐sensitive self‐assembly and CPL activity ( Figure 2.18) [48]. Guided by the Pt–Pt, π – π stacking, and dipole–dipole interactions, complex 59only self‐assembled into a non‐helical structure in chloroform, but formed helical fibers after a slow self‐assembly process (c. 200 minutes) in toluene. Interestingly, the UV–vis, PL, CD, and CPL performance were highly related to the chiral self‐assembly process. For an individual complex, no obvious CD and CPL signals were observed. After self‐assembly, however, 59exhibited both AIEE and AICPL features in toluene, with high g lumof ±1.0 × 10 −2(535 nm).
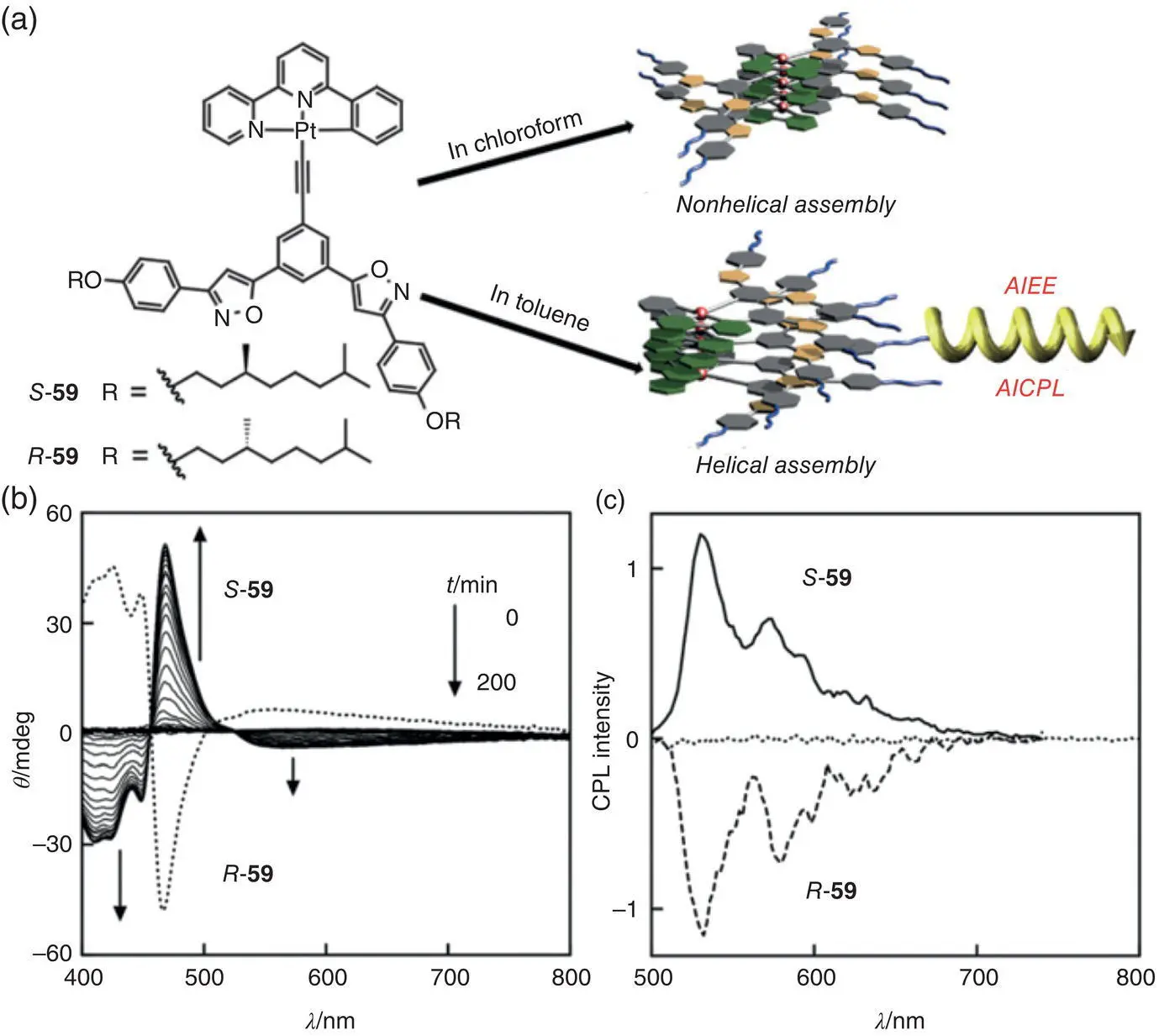
Figure 2.18 (a) Molecular structures of R ‐ 59and S ‐ 59and schematic illustration of their self‐assembly in chloroform and in toluene. (b) CD spectra of R ‐ 59(dotted line) and S ‐ 59(solid line) after self‐assembly in toluene at 25 °C. (c) CPL spectra of R ‐ 59(dashed line) and S ‐ 59(solid line) after self‐assembly in toluene at 25 °C.
Source: Reproduced with permission [48]. Copyright 2015, The Royal Society of Chemistry.
Recently, natural and/or artificial chiral templates are used in the fabrication of AICPL materials. In 2019, Ding and coworkers utilized the co‐assembly of DNA (chiral template) and carbazole‐based biscyanine (molecule 60, achiral luminophore) to fabricate CPL‐active materials ( Figure 2.19) [49]. In contrast to the conventional DNA‐binding dyes, the AIE‐active luminophore 60showed highly enhanced fluorescence after binding to the minor groove of DNA due to the restriction of intramolecular rotation. Induced CPL signals with | g lum| of 1.7 × 10 −3(550 nm) were observed for the DNA‐cyanine complexes, and the sign of CPL could be tuned by the chirality of DNA templates. Interestingly, the DNA‐cyanine complexes showed variable CPL upon multiple cycles of annealing. Recently, Zheng’s group and coworkers prepared a series of TPE macrocycle diquaternary ammoniums 61– 64( Figure 2.20a and b), which are associated with DNA via electrostatic interactions and exhibited chiroptical properties [50]. Their CPL performance was highly dependent on the position ( cis ‐ or gem ‐position) of the macrocycle. The mixture of cis ‐compounds and DNA exhibited strong CPL around 530 nm with g lumup to +2.8 × 10 −2, whereas the gem ‐compounds/DNA complexes showed nearly no CPL signals ( Figure 2.20c and d). The results demonstrated that the restriction of double‐bond rotation via cis ‐position cyclization played an important role in the generation of induced CPL.
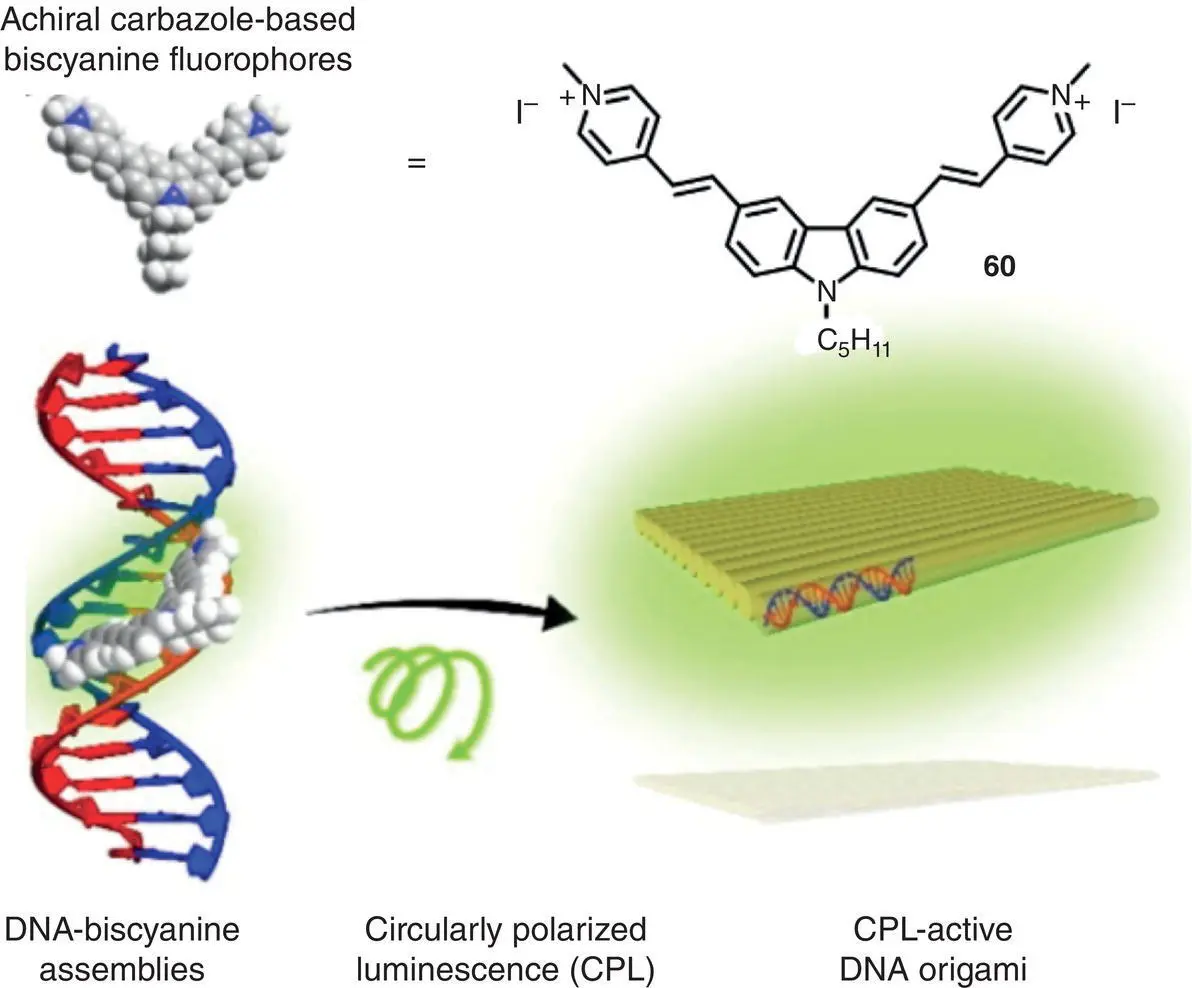
Figure 2.19 Schematic illustration of DNA‐biscyanine hybrid CPL‐active materials.
Source: Reproduced with permission [49]. Copyright 2019, American Chemical Society.
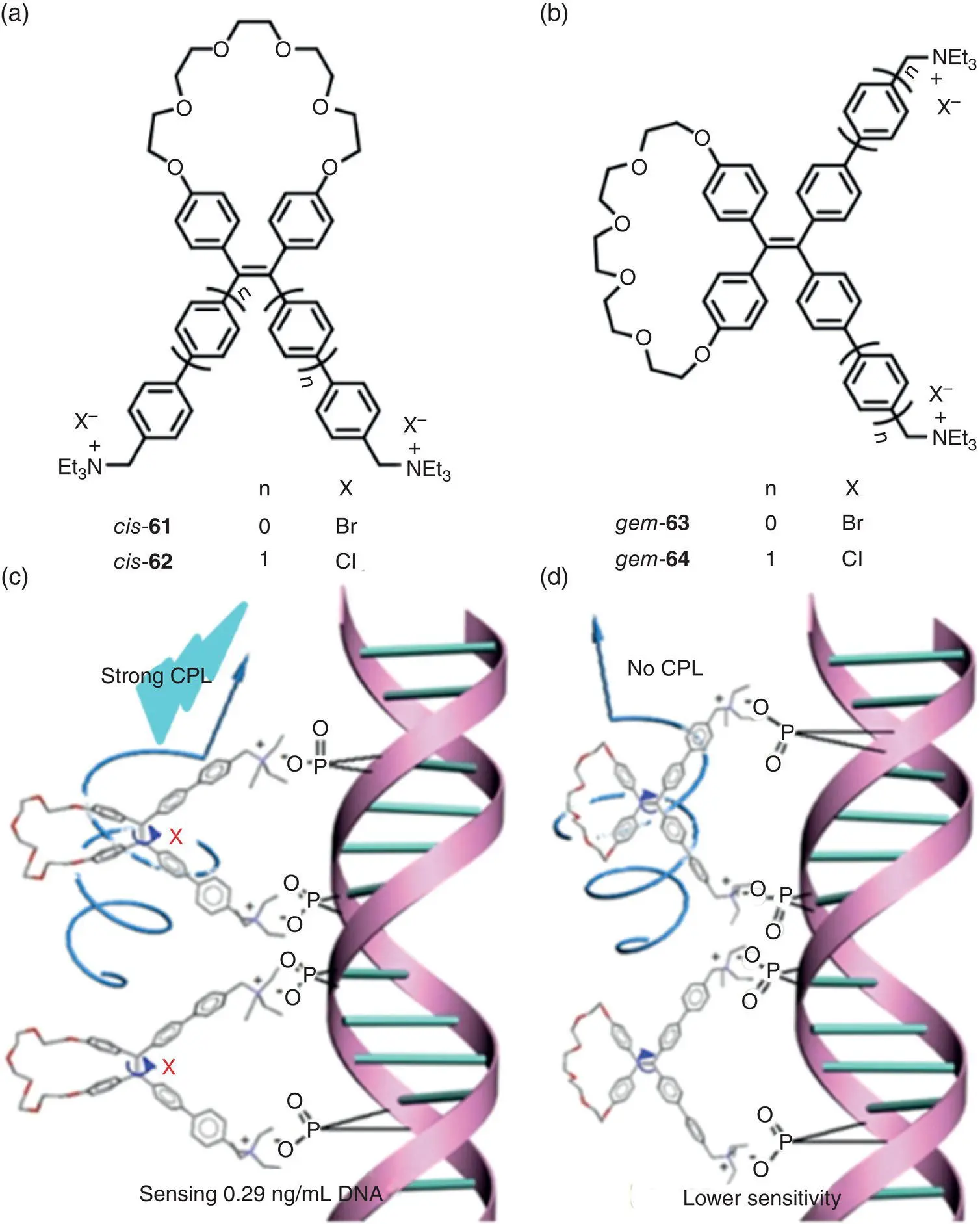
Figure 2.20 Molecular structures of TPE macrocycle diquaternary ammoniums (a) cis ‐ 61– 62and (b) gem ‐ 63– 64. Schematic illustration of the generation of CPL for (c) cis ‐ 62and (d) gem ‐ 64.
Читать дальше


















