In 2019, Chen et al. reported a novel AIEgen 31( Figure 2.5) with unique mechanoluminescence activities, which was rarely found in AIE‐active enantiomers [24]. With the existence of point chirality, 31exhibited intense CPL centered at 479 nm with g lumof +3.4 × 10 −3and −3.7 × 10 −3for R ‐ 31and S ‐ 31, respectively.
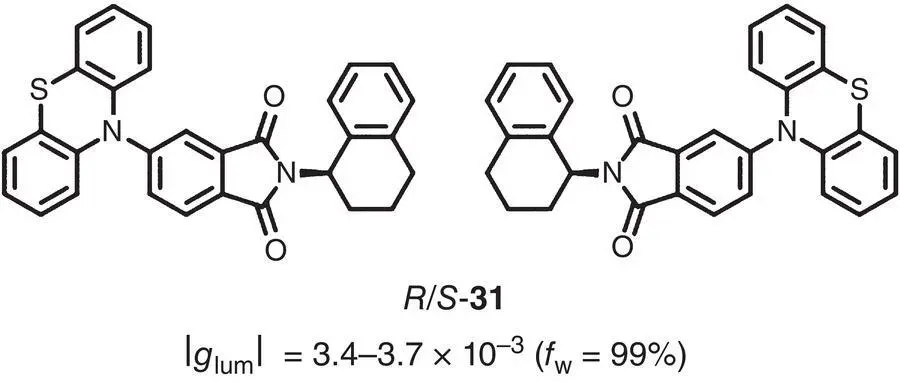
Figure 2.5 Molecular structures of chiral AIEgens R ‐ 31and S ‐ 31and corresponding g lum[24].
In 2019, Cai and coworkers synthesized three chiral TPE‐modified sulfono‐ γ ‐AApeptides, and one of them, molecule 32, was shown in Figure 2.6a [25]. Crystal structures indicated the presence of a helical scaffold of these molecules in an aqueous solution due to hydrogen bonding, which probably rendered the restriction of intramolecular rotations of TPE units ( Figure 2.6b–e). Following a well‐defined right‐handed helical scaffold, the luminescent TPE units were arranged in a helical fashion and exhibited CPL around 460 nm with g lumup to +1.2 × 10 −2. Later in 2020, the same group synthesized several TPE‐sulfono‐ γ ‐AApeptides with a left‐handed helix [26]. They found these helical foldamers emitted strong luminescence both in solution and in the aggregated state and can generate CPL with g lumup to +5.0 × 10 −3.
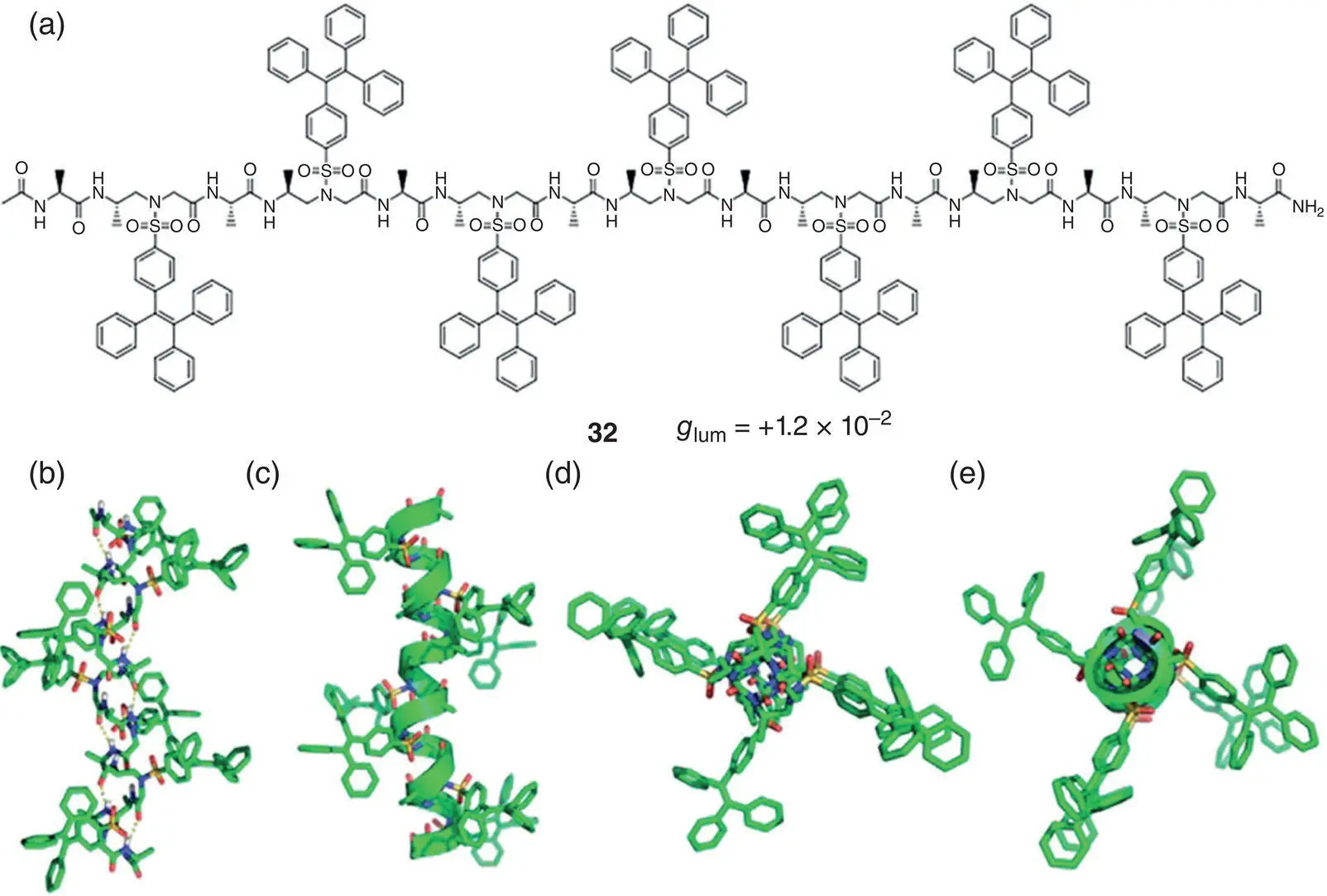
Figure 2.6 (a) Molecular structure of chiral AIEgen 32and corresponding g lum. (b–e) Crystal structure of molecule 32.
Source: Reproduced with [25]. Copyright 2019, American Chemical Society.
2.3 Macrocycles and Cages
Most of the typical AIE‐active molecules such as TPE, hexaphenylsilole (HPS), and their derivatives possess propeller‐shaped structures. In solution, these AIE luminophores are generally achiral due to the rapid conformational switching via intramolecular rotation. In the aggregated state, the intramolecular rotation is restricted; however, the random distribution of P ‐type and M ‐type conformations can only lead to chiroptical silence. Consequently, it becomes a significant challenge to produce CPL directly from simple AIE luminophores.
In 2016, Zheng et al. reported a pair of TPE tetracycles ( P ‐ 33and M ‐ 33) with stable chirality in a dilute solution ( Figure 2.7a) [27]. They prepared the tetracycles by intramolecular cyclization and successfully immobilized the propeller‐shaped conformation of the TPE unit. The helical configurations of the P and M enantiomers were found to be remarkably stable at room temperature and can be isolated by chiral high performance liquid chromatography (HPLC). Since the phenyl rings were fixed by the intramolecular linkers and thus prevented from rotation, the tetracycle showed strong luminescence even in solution. The quantum yield of 33reached up to 97% in a THF solution and 80% in the aggregated state. Mirror image CD and CPL spectra ( Figure 2.7b–d) were observed for the tetracycle enantiomers, with g lumof −3.3 × 10 −3(505 nm) and +3.1 × 10 −3(505 nm) for P ‐ 33and M ‐ 33, respectively, in a THF solution ( Figure 2.7c), and −5.0 × 10 −3(500 nm) and +6.2 × 10 −3(500 nm), respectively, in the aggregated state ( Figure 2.7d).
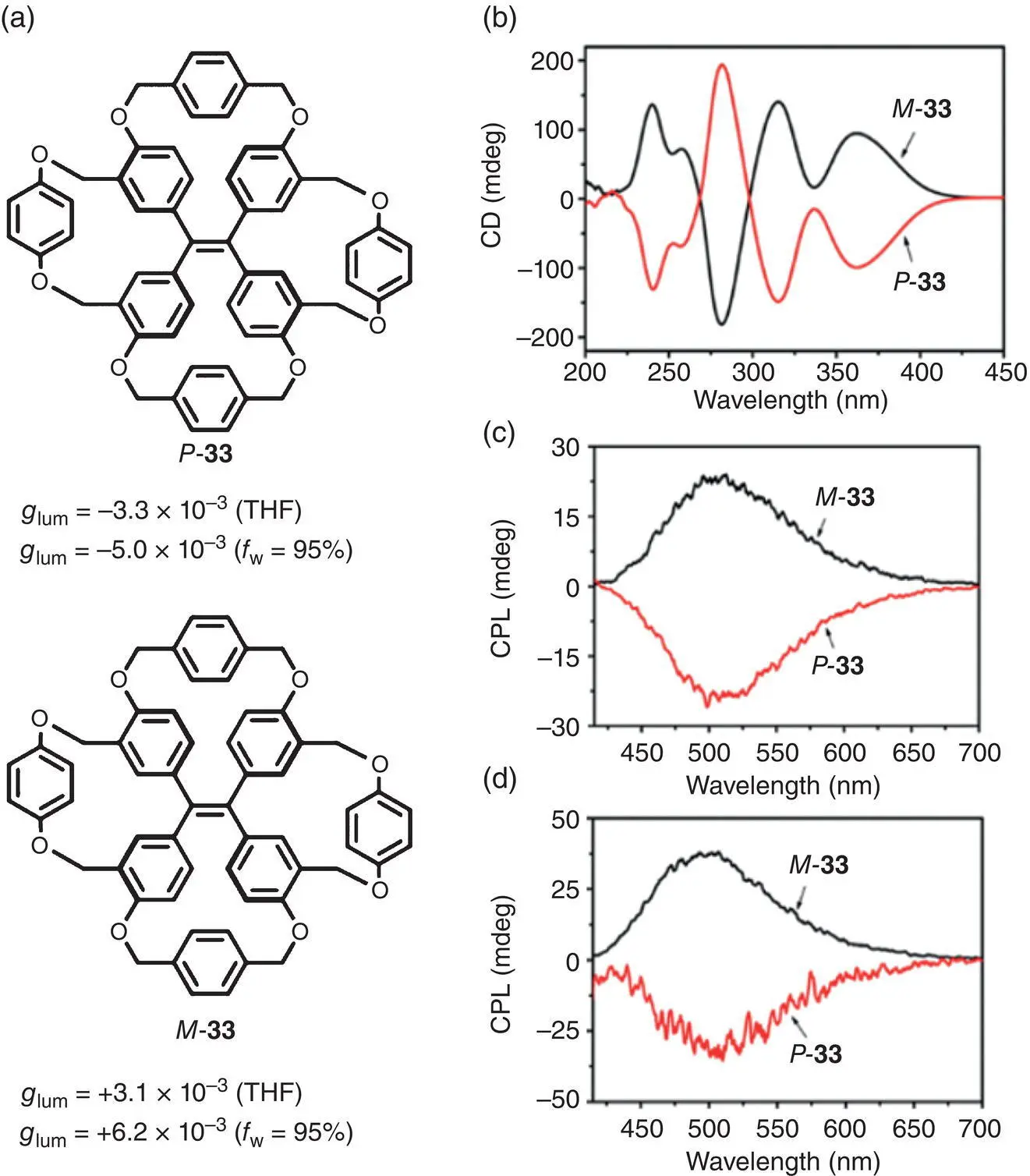
Figure 2.7 (a) Molecular structures of TPE tetracycle enantiomers P ‐ 33and M ‐ 33and corresponding g lum. (b) CD spectra and (c) CPL spectra of P ‐ 33and M ‐ 33in a THF solution (1.0 × 10 −3M). (d) CPL spectra of P ‐ 33and M ‐ 33in a THF/H 2O mixture ( f w= 90%).
Source: Reproduced with permission [27]. Copyright 2016, American Chemical Society.
In 2017, Cao’s group prepared two chiral cubic cages 34and 35by reversible covalent condensation based on a TPE derivative ( Figure 2.8) [28]. Each cage molecule was comprised of six TPE tetra‐aldehyde units and eight triaminoethylamine units. Unlike a single TPE molecule, the TPE units on each face of the cubic cage possessed stable configuration with P or M chirality. The rotation of the phenyl groups in the TPE units was restricted probably due to the strong steric hindrance from the neighboring TPE units. Therefore, the propeller‐shaped configurations of the TPE units were fixed in a cubic cage. By chiral HPLC, two pairs of enantiomers including homodirectional enantiomers (6 P ‐ 34and 6 M ‐ 34) and heterodirectional enantiomers (2 P 4 M ‐ 35and 4 P 2 M ‐ 35) were separated. Further characterizations showed that these cubic cages exhibited strong blue luminescence even in solution due to the rotation restriction of the benzene rings. Besides, the cage enantiomers exhibited mirror image CD and CPL spectra. The homodirectional cages (6 P ‐ 34and 6 M ‐ 34) showed a higher CPL activity at 450 nm with g lumof ±1.1 × 10 −3, and the heterodirectional cages (2 P 4 M ‐ 35and 4 P 2 M ‐ 35) revealed g lumof ±9.3 × 10 −4(450 nm).
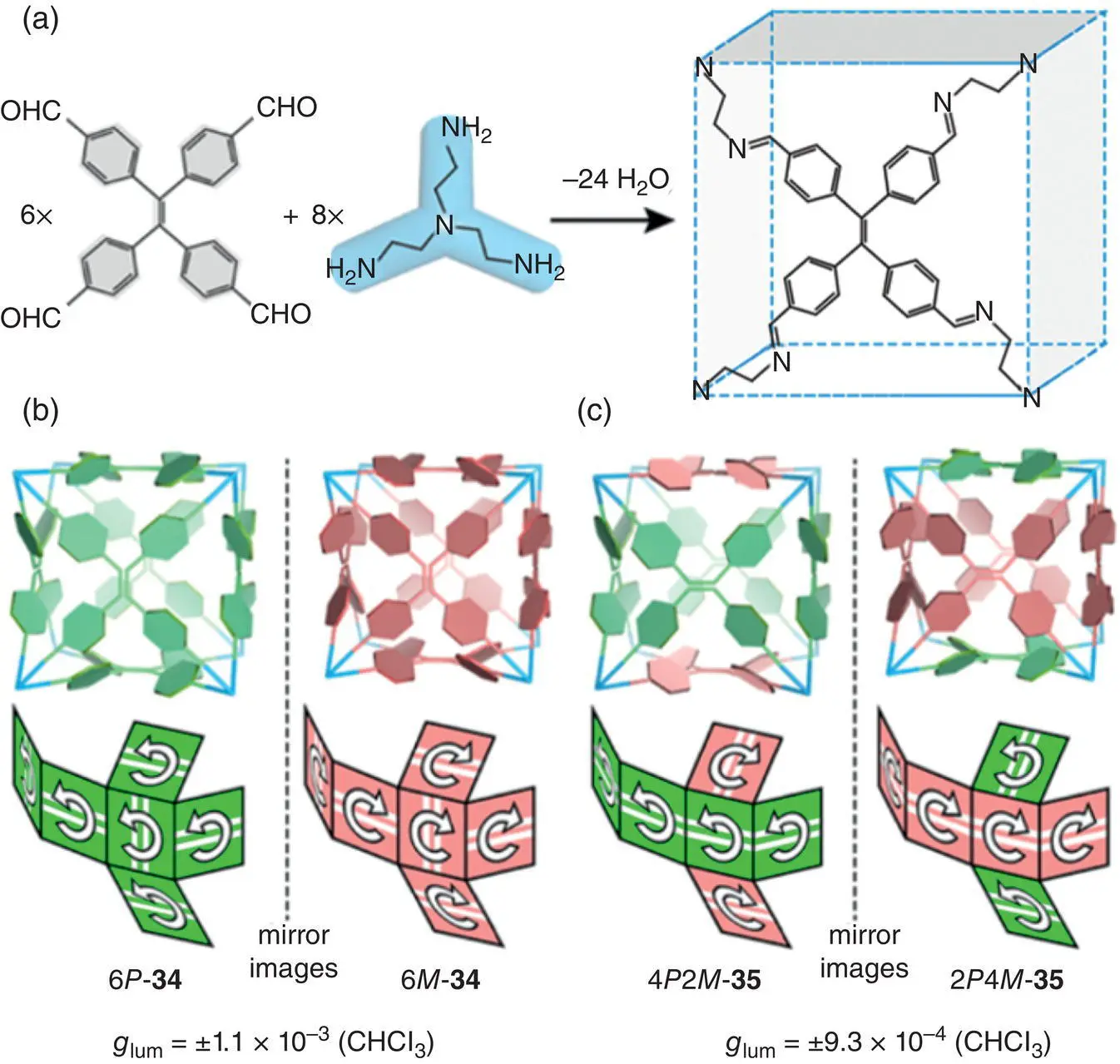
Figure 2.8 (a) Synthesis of organic cubes from six TPE tetra‐aldehyde units and eight triaminoethylamine units. Schematic representations of (b) homodirectional cubes 6 P ‐ 34and 6 M ‐ 34, and (c) heterodirectional cubes 4 P 2 M ‐ 35and 2 P 4 M ‐ 35, and corresponding g lum.
Source: Reproduced with permission [28]. Copyright 2017, American Chemical Society.
2.4 Metal Complexes and Clusters
Luminescent metal complexes also face the ACQ problem. Endowing metal complexes with AIE feature would greatly expand their applications in imaging and luminescent sensor. In 2018, Xiang’s group prepared a new class of chiral Pt(II)‐Salen complexes 36– 40( Figure 2.9) [29]. For all the complexes, nearly no emission was detected in dilute solutions of organic solvents such as CH 2Cl 2, benzene, MeCN, and THF, whereas they generated near‐infrared emission in THF/H 2O mixtures or in the solid state. With the absence of a traditional AIE‐active moiety, complexes 36– 40showed strong AIE activity. Theoretical calculations revealed that the binaphthyl unit quickly rotated in the solution and consequently caused luminescence quenching. In the aggregated state, the restriction of intramolecular motions (RIM) led to the near‐infrared emission. Complex 37showed AICPL when excited at 460 nm with g lumof ±5.0 × 10 −3(650 nm). Enantiopure 39also exhibited CPL at 655 nm with lower g lumof ±3.0 × 10 −3. The same group also prepared a series of chiral binuclear Pt(II) complexes in 2019 [30]. With binaphthyl quinoline as the bridge ligand, these complexes possessed helical structure, and exhibited CPL with high g lumup to ±1.0 × 10 −2(653–683 nm).
Читать дальше
















