Incorporating less emissive chiral moieties and AIE luminophores appeared to be an alternative and more flexible pathway to AICPL due to the facile synthesis and broader source of chiral precursors. In 2015, two pairs of chiral 1,8‐naphthalimide chromophores 4and 5with AIE activities were prepared by Cheng and coworkers [18]. In contrast to the previous examples ( 1– 3), the chiral moiety and chromophores of 4and 5were connected by flexible alkyl chains rather than rigid conjugated junctions. PL spectra demonstrated the AIE effect of 1,8‐naphthalimide in THF/H 2O mixtures. In a THF solution, nearly mirror image CPL signals centered at 462 nm could be observed with g lumof −6.1 × 10 −3and +5.5 × 10 −3for R ‐ 4and S ‐ 4, respectively. In THF/H 2O mixtures, CPL centered at 490 nm could be observed with lower g lumof +2.8 × 10 −3and −2.2 × 10 −3for R ‐ 4and S ‐ 4, respectively. It was found interestingly that the CPL signals became reversed in a pure THF solution and in THF/H 2O mixtures. The CPL spectra of 5exhibited similar phenomenon where the CPL signal reversed at various states with lower g lumthan 4. Calculation results demonstrated that the CPL signals may result from different conformations and dihedral angles at distinct states. Besides, the difference between 4and 5indicated that the length of alkyl chain may also influence the CPL performance.
In 2019, Jiang et al. prepared two boron difluoride complexes ( 6and 7) with red emission [19]. In spite of a small difference in structure, 6and 7exhibited dramatically different photophysical properties. The luminescence intensity of 6was not sensitive to the aggregation process, while 7showed typical AIEE effect in the aggregated state. In a dichloromethane (DCM) solution, 6showed CPL signals with | g lum| in the range of 1.3–1.6 × 10 −3(600 nm). Complex 7exhibited weak CPL with | g lum| of c. 10 −3(609 nm) in a THF solution. As a contrast, enhanced CPL with high | g lum| up to 1.6 × 10 −2(653 nm) was observed in the aggregated state. Besides, it was found interestingly that 7could be used as a CPL switcher by reversible protonation of the N , N ‐dimethylaminium groups. In 2019, Zhao et al. reported a series of R ‐BINOL‐derived CPL‐active AIE molecules 8– 13, which exhibited tunable luminescent properties [20]. With the various modifications on the BINOL skeleton, the corresponding CPL could be tuned with | g lum| in the range of 0.6–10 × 10 −3(518–617 nm).
CPL‐active AIE SOMs 1– 5were prepared by incorporating chiral binaphthyl moieties with AIEgens. This principle of molecular design successfully endowed the SOMs with both the CPL and AIE activities. However, the | g lum| of these molecules was still limited in a low range (<6 × 10 −3), presumably due to the insufficient association between the chiral moieties and AIEgens. Later in 2020, Qiu’s group synthesized a series of CPL‐active AIE molecules 14– 25based on helicenes ( Figure 2.2) [21]. The molecular structures were varied in linkage position, conjugation, length of linkage, and number of substituent. It was found that the linkage position played a dominating role in CPL activities. According to the CPL spectra of the suspension, all the 2‐ (or 2,15‐) substituted AIE‐helicene adducts (compounds 14– 19) exhibited obvious CPL signals, while all the 4‐ (or 4,13‐) substituted molecules (compounds 20– 25) appeared to be nearly CPL silent. Theoretical calculations indicated that this phenomenon was probably due to the different distribution of various rotational conformers in the aggregated state. The existence of favored conformations for 2‐ (or 2,15‐) substituted AIE‐helicene adducts leads to CPL‐active properties. On the contrary, the coexistence of various rotational conformers for 4‐ (or 4,13‐) substituted analogs results in a cancellation of CPL signals. Controlling the conjugation between the helicene moieties and AIE luminophores could easily tune the emission color. Additionally, shortening the length of linkage or increasing the number of substituents were proved to be efficient ways to enhance the CPL performance, especially for | g lum| (up to 1.5 × 10 −2).
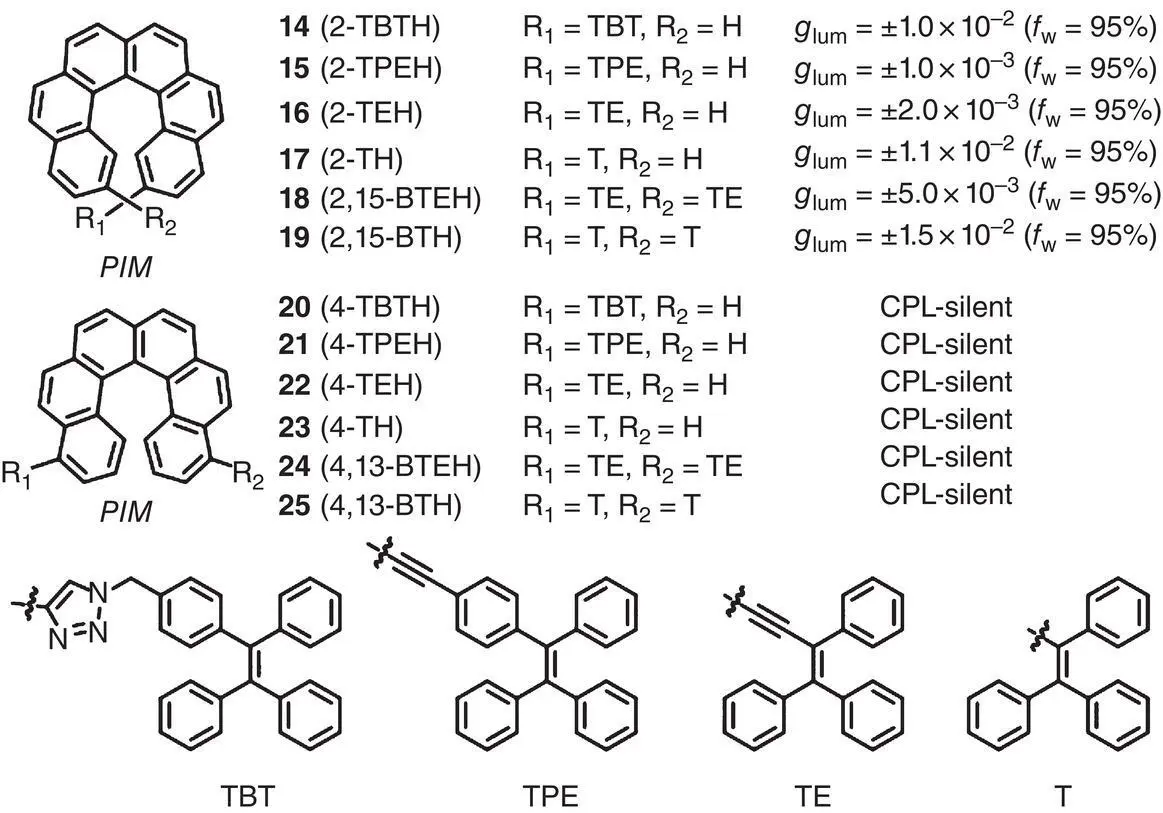
Figure 2.2 Molecular structures of chiral AIEgens 14– 25and corresponding g lum[21].
In 2018, Tang’s group prepared a series of BINOL‐derived chiral AIEgens (molecules 26– 29, Figure 2.3) with AIE and delayed fluorescence properties [22]. In a toluene solution, these molecules exhibited obvious mirror image CPL signals with | g lum| in the range of 0.5–1.2 × 10 −3. Besides, 26– 29were used as emitting layers in CP‐OLED devices, and exhibited external quantum efficiency up to 9.3 and 3.5% with dissymmetry electroluminescence factor ( g EL) as high as +0.026/−0.021 and +0.06/−0.06 for the doped film and the neat film, respectively.
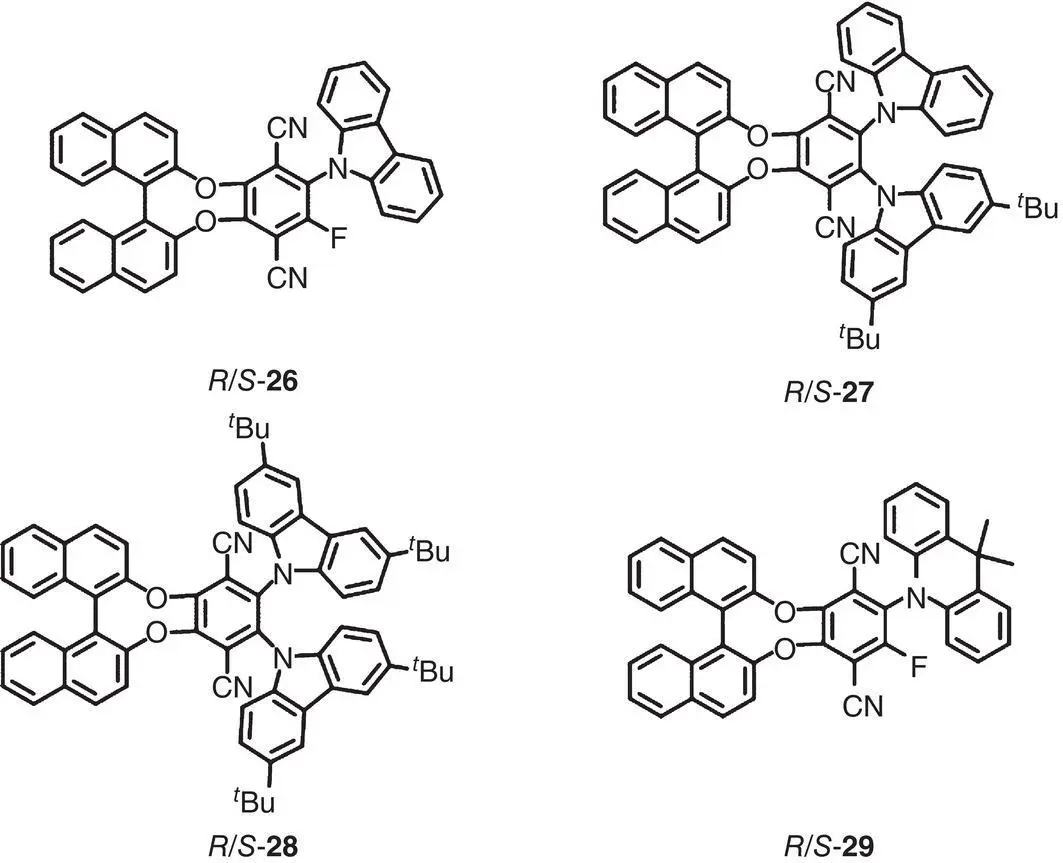
Figure 2.3 Molecular structures of chiral AIEgens 26– 29[22].
In 2019, Ye et al. prepared a novel chiral AIEgen 30through the incorporation of a chiral BINOL moiety and two TPE units via Suzuki reaction ( Figure 2.4a) [23]. 30exhibited a typical AIE feature and showed bright yellow luminescence in the aggregated state ( Figure 2.4b). CPL spectra demonstrated that the enantiomers generated mirror image CPL signals centered at 532 nm in the aggregated state ( f w= 99%) with g lumof −2.7 × 10 −3and +2.8 × 10 −3for R ‐ 30and S ‐ 30, respectively. In the spin‐coated film, CPL was observed at 532 nm with higher g lumof −3.2 × 10 −3and +3.6 × 10 −3for R ‐ 30and S ‐ 30, respectively ( Figure 2.4c). Besides, R ‐ 30and S ‐ 30were also used as CP‐OLED emitters for nondoped device and showed CPL centered at 534 nm with g ELof −3.0 × 10 −3and +3.2 × 10 −3.
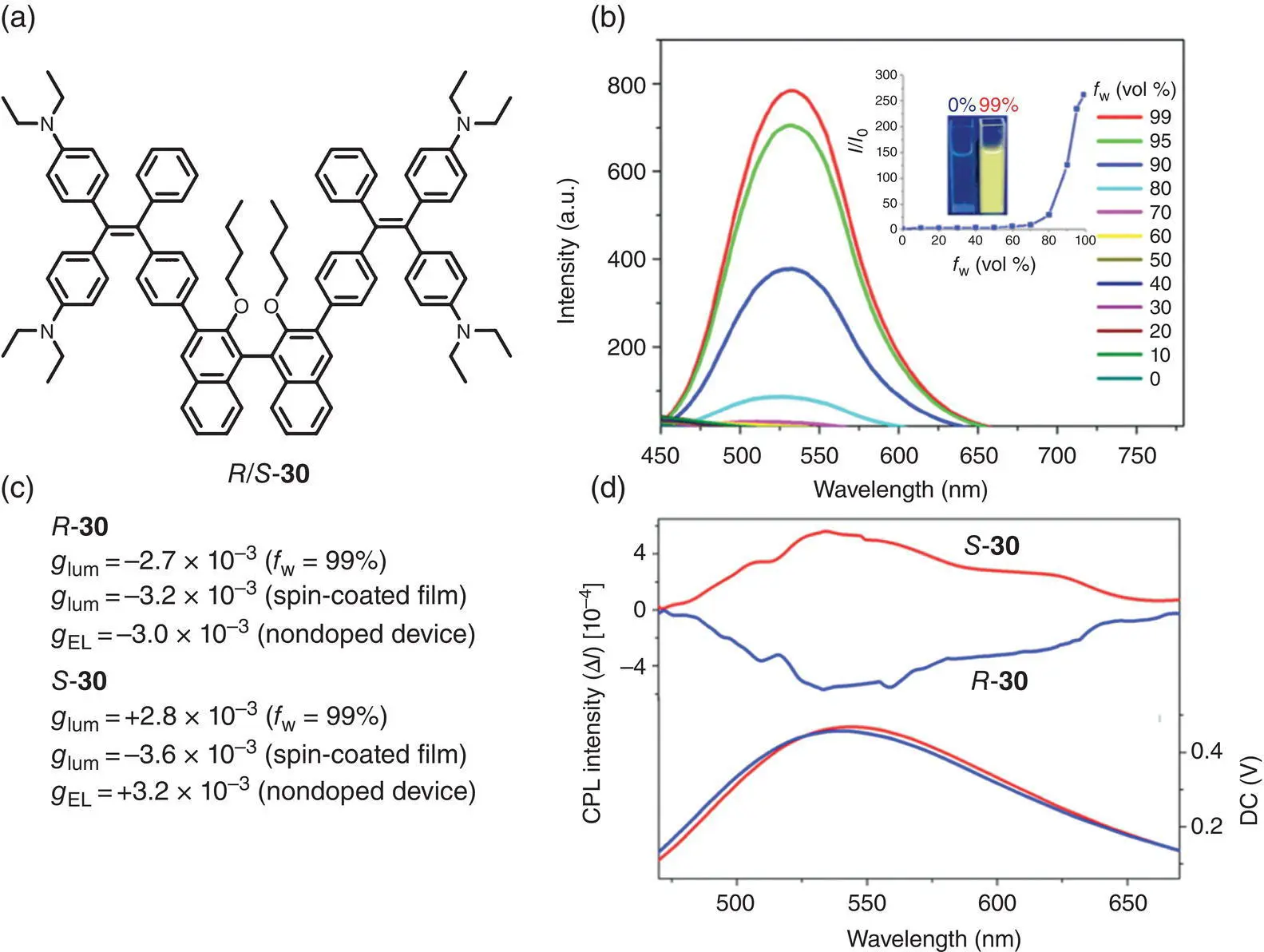
Figure 2.4 (a) Molecular structure of chiral AIEgen enantiomers 30and corresponding g lum. (b) PL spectra of 30in various THF/H 2O mixtures. (c) CPL spectra of R ‐ 30and S ‐ 30in spin‐coated films.
Source: Reproduced with permission [23]. Copyright 2019, American Chemical Society.
Читать дальше















