2 Circularly Polarized Luminescence of Aggregation‐induced Emission Materials
Fuwei Gan, Chengshuo Shen, and Huibin Qiu
School of Chemistry and Chemical Engineering, Frontiers Science Center for Transformative Molecules, State Key Laboratory of Metal Matrix Composites, Shanghai Jiao Tong University, Shanghai, China
2.1 Introduction of Circularly Polarized Luminescence
Electronic circular dichroism (ECD) measures the differential molar absorption coefficient (Δε) between a left‐handed circularly polarized light (ε L) and a right‐handed circularly polarized light (ε R) for electronic transitions:
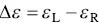
ECD is the chiroptical counterpart of UV–vis absorption and is utilized extensively in most chiral systems including small molecules, biomolecules, supramolecular assemblies, polymers, liquid crystals (LCs), etc., providing valuable information of the ground states.
For a luminescent chiral system, one can investigate its circularly polarized luminescence (CPL), which measures the differential emission (Δ I ) between a left‐handed circularly polarized light ( I L) and a right‐handed circularly polarized light ( I R):
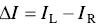
Unlike ECD measurement, determination of the absolute emission intensity of CPL is quite difficult, and it is customary to quantify the intensity difference using its relative value, namely, luminescence dissymmetry factor ( g lum):

The value of g lumshould be limited between −2 and +2, and 0 represents the absence of circularly polarized emission. Similar to its absorption analog ECD, CPL reflects the information of the excited states of a chiral system.
Studies on CPL have lasted for a long history and the first observation of CPL was recorded in 1948 by Samoilov from chiral Na[UO 2(CH 3COO) 3] crystals [1]. From 1960s to 1970s, CPL of chiral small organic molecules (SOMs) and chiral lanthanide complexes were reported occasionally [2–4]. Such slow progress was attributed to the factor that all the CPL analyses were performed on custom‐made instruments. Until recently, CPL spectrometers have become commercially available, and CPL‐related research starts to experience a fast development.
Generally, systems with intrinsically chiral luminophores or luminophores situated in a chiral environment can generate CPL signals. However, due to the detection limit, most early reported examples were restricted to lanthanide complexes since their f – f Laporte‐forbidden transitions can give relatively high CPL activities ( g lum~ 10 −2to 10 −1) [5]. This situation is changing recently following the discovery of several categories of SOMs with intense CPL [6–8], such as chiral ketones, cyclophanes, binaphthyl derivatives, helicenes, and boron dipyrromethenes (BODIPYs), and their promising applications in circularly polarized organic light‐emitting diodes (CP‐OLEDs), circularly polarized organic semiconductor transistor, and other optoelectronic devices [9–12].
For CPL measurements, the most examples were done in dilute solutions, while for devices, materials need to generate CPL in the aggregated state. For the most luminophores, severe π – π stacking can be observed in the aggregated state, resulting in strong luminescence quenching and hence, less or even no emission. Aggregation‐induced emission (AIE) creates a highly efficient way to solve the aggregation‐caused quenching (ACQ) problem. To construct aggregation‐induced circularly polarized luminescence (AICPL) materials, one can introduce nonemissive chiral moieties into an AIE system, rendering the AIEgens chiral, or in several cases, chiral luminophores linked together with AIEgens can also give an efficient AICPL performance. Besides, constructing a chiral environment using supramolecular chemistry or placing AIEgens in chiral polymers or LCs is emerging as another practical way to build AICPL systems.
Tang and Wong et al. have previously summarized the strategies for the fabrication of AICPL materials that were developed by mid‐2016 [13]. In this chapter, we have collected the most recently reported AICPL systems and classified them into six categories according to the different features: (i) SOMs, (ii) macrocycles and cages, (iii) metal complexes and clusters, (iv) supramolecular systems, (v) polymers, and (vi) LCs.
2.2 Small Organic Molecules
SOMs play an important role in CPL materials. However, most of them suffer from a severe ACQ effect, which largely limits the further applications. To solve the ACQ problem in the aggregated state, several strategies have been applied in designing novel CPL‐active molecules. Among all the strategies, introducing AIE‐active moieties (such as tetraphenylethene, TPE) into chiral luminophores is one of the most useful methods. In 2014, Moya’s group reported a CPL‐active organic molecule with a novel skeleton, in which BODIPY acted as the luminophore moiety and 1,1′‐bi‐2,2′‐naphthol (BINOL) functioned as the chirality perturbing moiety [14]. Later in 2015, covalent modification based on the luminescent BODIPY–BINOL skeleton by introducing two TPE units via Sonogashira reaction (molecule 1) was reported by Zhu et al. ( Figure 2.1) [15]. Photoluminescence (PL) spectra in CH 2Cl 2/hexane mixtures demonstrated that molecule 1has a typical AIE feature where the luminescence intensity strongly increased following the addition of hexane. In addition, upon varying the hexane fraction, CPL of molecule 1remained nearly constant with g lumof c. ±2 × 10 −3(630 nm). In 2017, Cheng and coworkers synthesized another AICPL molecule 2based on a diboron core and two TPE pendants [16]. Molecule 2showed interesting photophysical properties in the different mixed solvents upon aggregation. For instance, 2exhibited aggregation‐induced emission enhancement (AIEE) effect in CH 2Cl 2/hexane mixtures, and | g lum| (absolute value of g lum) varied in the range of 2.7–5.4 × 10 −4(550 nm). Meanwhile, it revealed an ACQ feature in tetrahydrofuran (THF)/H 2O mixtures, and | g lum| was lower than 5.5 × 10 −4(550 nm). The above two examples demonstrated a simple molecular design using chiral luminophores and AIEgens linked together by π ‐conjugation. However, this strategy is limited by the tedious synthesis of the chiral chromophores and the relatively low | g lum|. In 2018, Tang and coworkers synthesized molecule 3by incorporating TPE units and a BODIPY dye through a single bond [17]. By adding water into a THF solution, 3exhibited tunable emission from green to yellow. Besides, mirror image CPL signals with | g lum| up to 4.1 × 10 −3(525 nm) were observed.
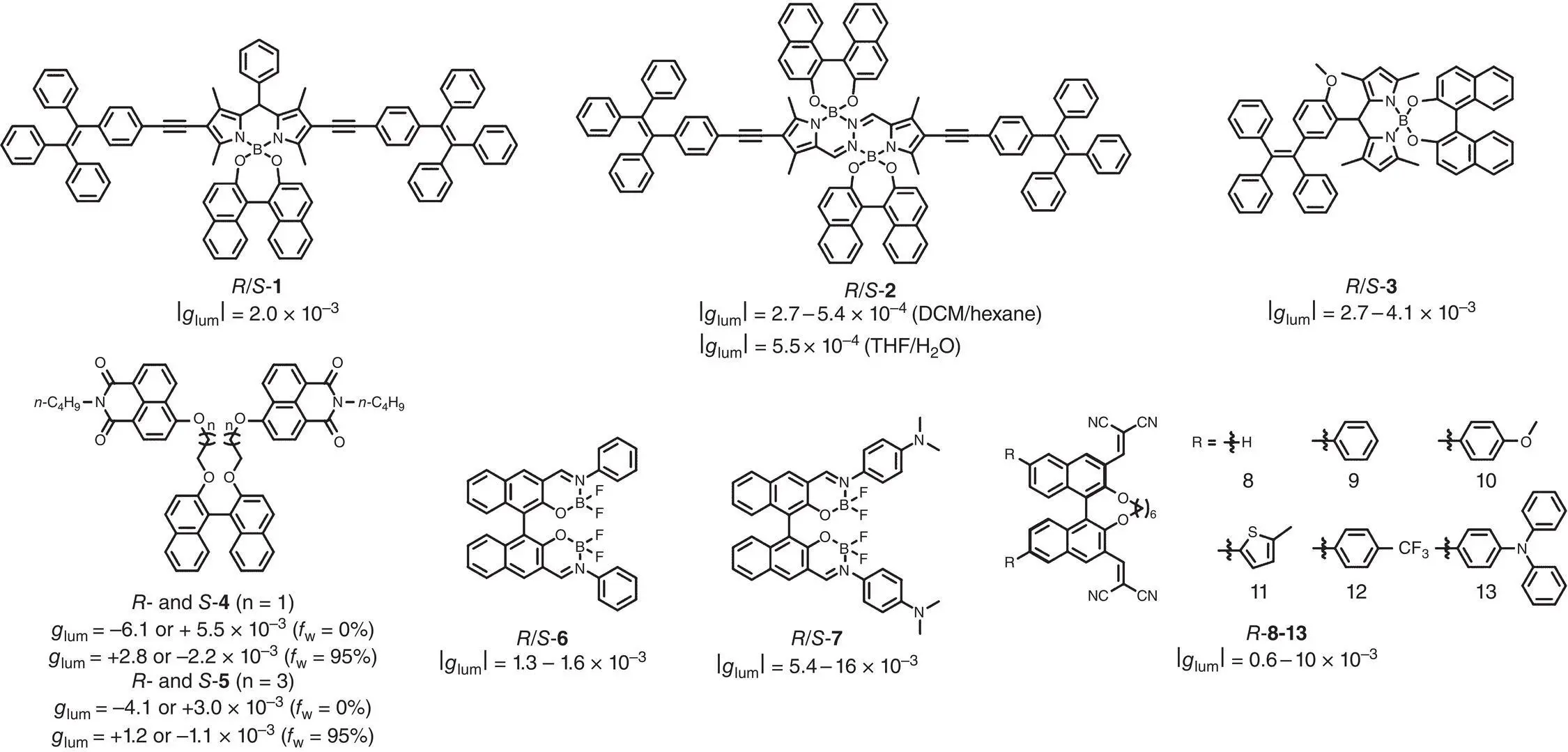
Figure 2.1 Molecular structures of chiral AIEgens 1– 13and corresponding g lum( f windicate the fraction of water in the solvent mixture) [15–20].
Читать дальше