Source: Reproduced with permission [50]. Copyright 2020, American Chemical Society.
In addition to the AIEgen‐DNA complexes, in 2017, Liu et al. reported a co‐assembly system with promising CPL activity based on a chiral gelator 65and several achiral AIEgens ( Figure 2.21) [51]. Compound 65self‐assembled into nanotube‐like structures in a DMSO/H 2O mixture (1 : 1). Without the addition of AIEgens, no emission can be observed due to the lack of chromophores. After doping with the achiral AIEgens, the resulting gel exhibited tunable CPL (425–595 nm) with | g lum| in the range of 0.2–1.7 × 10 −3. In 2019, Yin et al. adopted a similar strategy to construct CPL‐active materials by the cogelation of a chiral glutamic acid‐containing gelator 66and an achiral AIEgen 67( Figure 2.22) [52]. Under D ‐ 66/ 67= 100 : 1, left‐handed CPL signal around 510 nm was observed with g lumof +1.1 × 10 −2. However, the CPL signal inversed ( g lum= −1.5 × 10 −2, 520 nm) at D ‐ 66/ 67= 16 : 1, mainly due to the competition of distinct packing modes in the hydrogen bonding‐driven co‐assembly.
In 2020, Liu’s group developed a CPL‐active supramolecular system based on cyclodextrin‐metal‐organic framework ( γ CD‐MOF) and achiral luminophores, including traditional organic dyes and AIE luminophore [53]. Through host–guest interactions, the chiral space of γ CD‐MOF could be utilized for CPL induction of achiral luminophores. The authors emphasized the importance of the ordered packing of the achiral luminophores in γ CD‐MOF for the induction of CPL, since the amorphous luminophores and γ CD mixture exhibited nearly no CPL signals. For the crystalline luminophores@ γ CD‐MOF materials, the result was quite different. As for the AIE luminophore@ γ CD‐MOF system, CPL centered at 500 nm was observed with g lumof −2 × 10 −3. In 2020, Tang et al. developed a novel CPL‐active system comprised of achiral AIEgen and chiral crystalline poly(L‐lactide) (PLLA) [54]. The confinement of AIEgen in the semicrystalline PLLA film endowed the resulting film with CPL activity with | g lum| of 1.6 × 10 −3(540 nm).
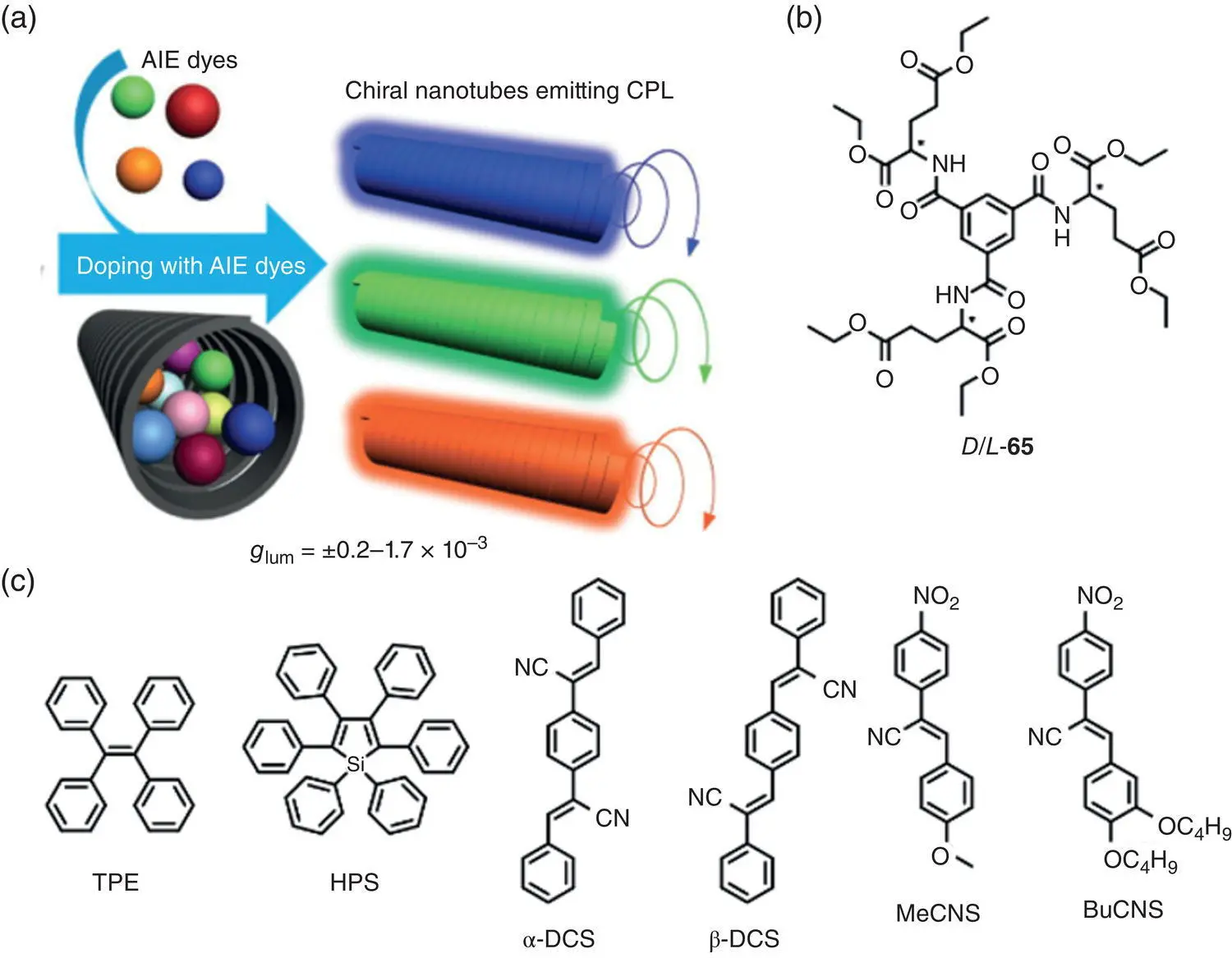
Figure 2.21 (a) Schematic illustration of the co‐assembly processes. (b) Molecular structure of chiral gelator 65. (c) Molecular structures of achiral AIEgens.
Source: Reproduced with permission [51]. Copyright 2019, Wiley‐VCH.
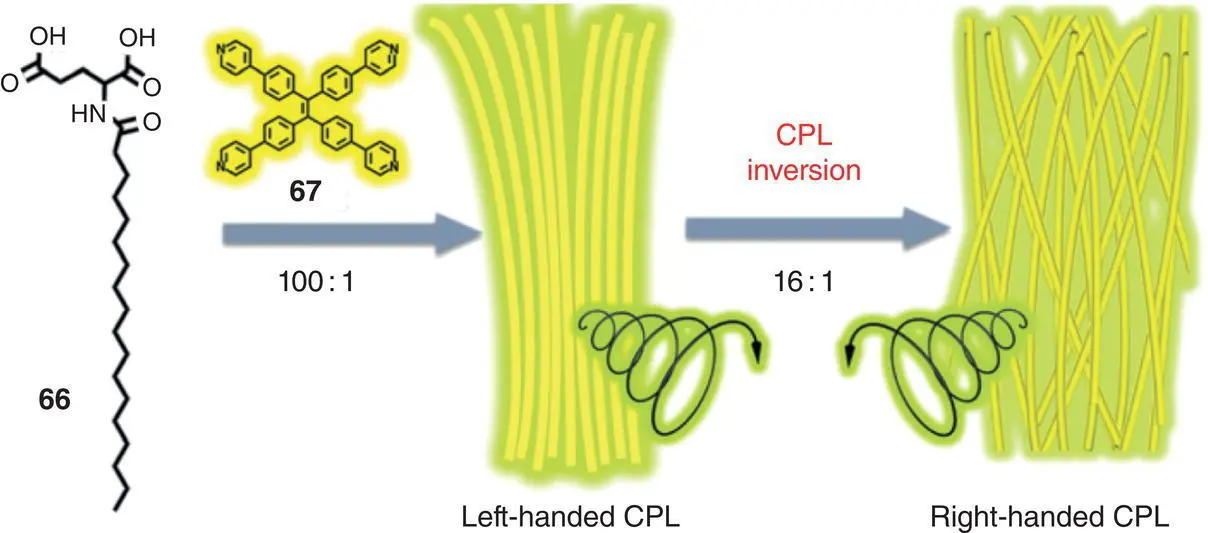
Figure 2.22 Schematic illustration of the co‐assembly of molecules 66and 67under different molar ratio.
Source: Reproduced with permission [52]. Copyright 2019, The Royal Society of Chemistry.
In 2019, Tang’s group reported a unique CPL‐active supramolecular system based on the co‐assembly of a chiral BINOL‐derived gold complex 68( Figure 2.23a) and three achiral luminophores ( Figure 2.23b) [55]. Chiral complex 68formed helical fibers through a long‐time self‐assembly process, and the chirality was controlled by the chirality of the gold complex. R ‐ 68formed M ‐type helical fibers, while S ‐ 68formed P ‐type helical fibers. However, no obvious CPL signal was observed for these helical fibers. Interestingly, the co‐assembly systems comprised of the chiral gold complex and achiral luminophores exhibited strong CPL signals with different colors (410–560 nm) and relatively high g lumup to 5 × 10 −3( Figure 2.23c). In 2019, Han and coworkers prepared CPL‐active AIEgen‐silica hybrid hollow nanotubes through a spontaneous chiral self‐assembly process in the absence of symmetry‐breaking agent [56]. These nanotubes revealed a helical structure and exhibited CPL around 450 nm with | g lum| up to 2 × 10 −2.
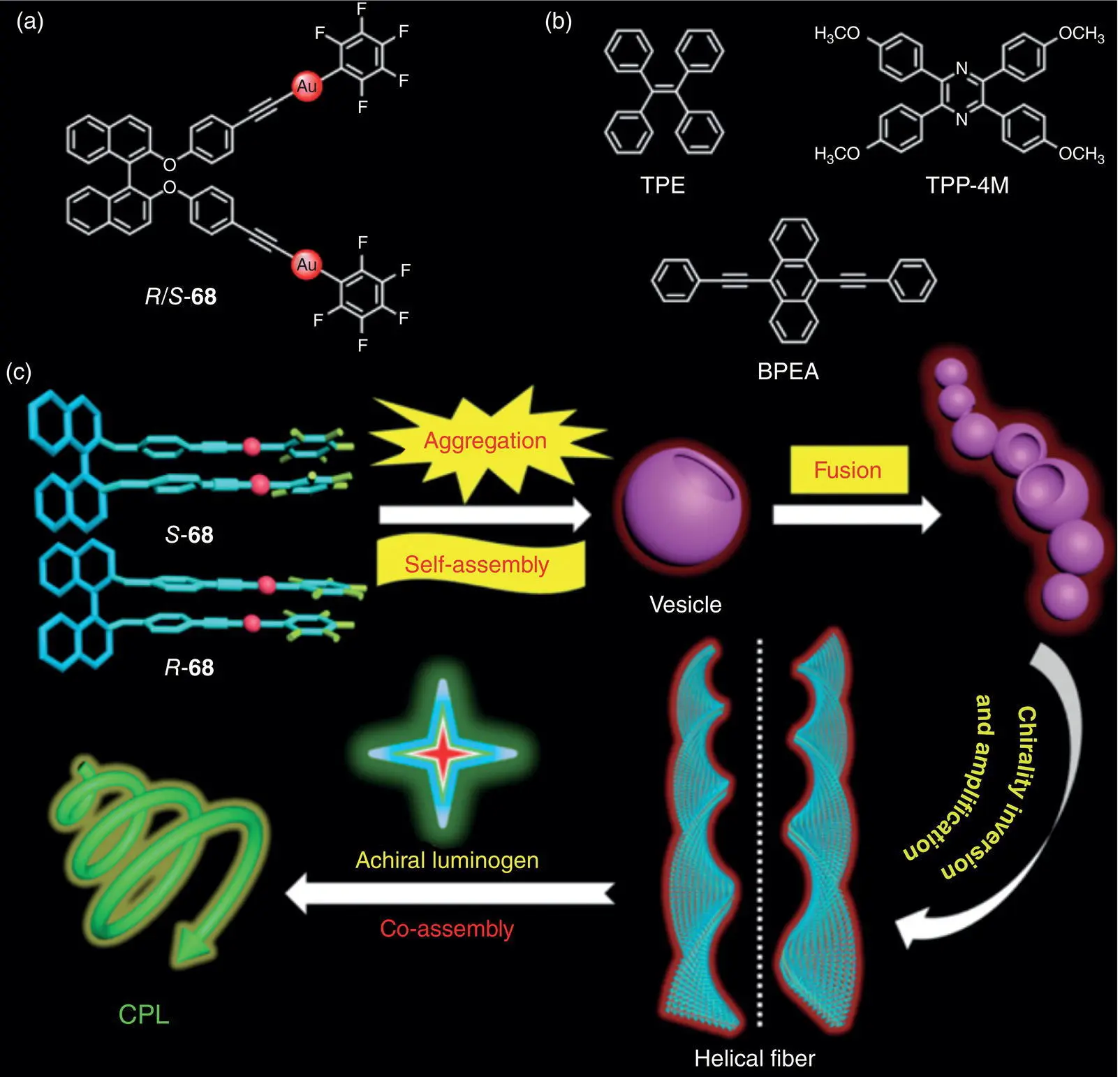
Figure 2.23 (a) Molecular structures of chiral gold complex enantiomers R ‐ 68and S ‐ 68. (b) Molecular structures of achiral luminophores TPE, 2,3,5,6‐tetrakis(4‐methoxyphenyl)pyrazine (TPP‐4M), and 9,10‐bis(phenylethynyl)anthracene (BPEA). (c) Schematic illustration of the hierarchical self‐assembly and co‐assembly processes.
Source: Reproduced with permission [55]. Copyright 2019, American Chemical Society.
The development of chiral AIE‐based supramolecular systems greatly improved the CPL performance and expanded the family of AICPL materials. Besides, the properties of the resulting CPL‐active materials were highly tunable via modulation of the supramolecular interactions.
Compared to the small molecule and supramolecular systems, polymers possess distinctive advantages, such as highly stable and readily processible. In 2013, Zhu et al. prepared the first conjugated polymer 69with AICPL by incorporating difunctional TPE units and tyrosine‐derived pendants via Sonogashira reaction ( Figure 2.24) [57]. The resulting polymer was nearly nonemissive in a THF solution, but appeared to be strongly luminescent upon the addition of water, indicating a typical AIE feature. It revealed obvious CPL around 500 nm both in solution and in the aggregated state, and the g lumcan be tuned from +0.08 to +0.44 by changing the content of water.
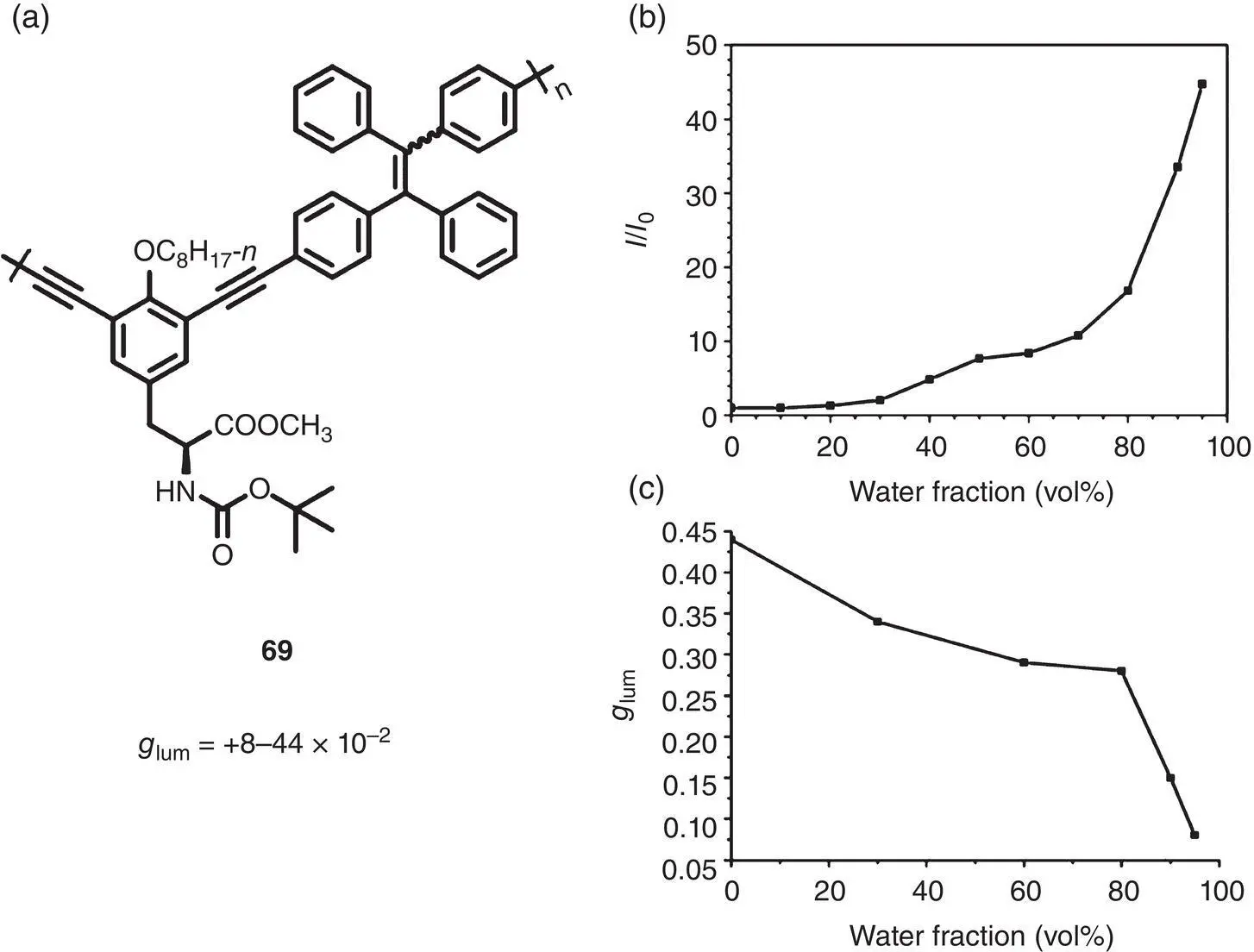
Figure 2.24 (a) Molecular structure of chiral TPE‐containing polymer 69and corresponding g lum. (b) Plot of ( I / I 0) values versus water fraction of polymer 69in THF/H 2O mixtures (1.0 × 10 −5M). (c) CPL dissymmetry factor g lumversus water fraction of polymer 69in THF/H 2O mixtures (1.0 × 10 −5M).
Source: Reproduced with permission [57]. Copyright 2013, The Royal Society of Chemistry.
In 2015, Zhu and coworkers synthesized a series of chiral conjugated polymers ( 70– 73) with AIE activity via Sonogashira and Suzuki reactions ( Figure 2.25) [58]. R ‐1,1′‐binaphthyl group and the TPE unit were introduced into the main chain as chiral moiety and AIE‐active moiety, respectively. The chiral conjugated polymers 70– 73all showed strong luminescence and obvious CD signals. However, only 70exhibited detectable CPL signals around 530 nm in the aggregated state with g lumof −1.6 × 10 −3. Further morphology characterization showed that polymer 70formed right‐handed helical nanostructure in the THF/H 2O mixture, which was consistent with the negative CPL signal. In 2018, another two examples of chiral AIE‐active conjugated polymers 74and 75were prepared by Cheng et al. via click chemistry and Suzuki cross‐coupling reactions ( Figure 2.25) [59, 60]. Polymer 74exhibited weak luminescence and nearly no CPL signal in a THF solution. When f wof the THF/H 2O mixture exceeded 40%, AICPL could be easily observed and g lumreached up to −5.6 × 10 −3(around 500 nm) and +7.0 × 10 −3(around 500 nm) at f w= 90% for R ‐ 74and S ‐ 74, respectively. Polymer 75exhibited CPL with g lumof −1.3 × 10 −3(496 nm) and +1.1 × 10 −3(496 nm) for R ‐ 75and S ‐ 75, respectively, in the spin‐coated film. R ‐ 75and S ‐ 75were also used in doping‐free electroluminescent devices, which showed CPL centered at 505 nm with higher g ELof −1.9 × 10 −2and +2.4 × 10 −2, respectively.
Читать дальше
















