1 1 Momke S, Fink S, Wohlke A, Drogemuller C and Distl O. Linkage of bilateral convergent strabismus with exophthalmus (BCSE) to BTA5 and BTA18 in German Brown cattle. Anim Genet 2008; 39(5): 544–549.
2 2 Scacco G, Rambaldi AM, Roccaro M, et al. Bilateral convergent strabismus with exophthalmos in cattle ‐ what do we know? Large Anim Rev 2017; 23(2): 67–71.
3 3 Fink S, Momke S and Distl O. PLXNC1 and RDH13 associated with bilateral convergent strabismus with exophthalmus in German Brown cattle. Mole Vis 2012; 18(235–36): 2229–2240.
4 4 Momke S and Distl O. Bilateral convergent strabismus with exophthalmus (BCSE) in cattle: an overview of clinical signs and genetic traits. Vet J 2007; 173(2): 272–277.
5 5 Regan WM, Gregory PW and Mead SW. Hereditary strabismus in Jersey cattle. J Hered 1944; 35(8): 233–234.
6 6 Holmes JR and Young GB. A note on exophthalmos with strabismus in shorthorn cattle. Vet Rec 1957; 69: 148–149.
7 7 Julian RJ. Bilateral divergent strabismus in a Holstein calf. Vet Med Small Anim Clin 1975; 70(10): 1151.
8 8 Distl O and Scheider A. An unusual eye defect in Highland cattle: diverging unilateral strabismus. Dtsch Tierarztl Wochenschr 1994; 101(5): 202–203.
9 9 Distl O and Gerst M. Association analysis between bilateral convergent strabismus with exophthalmus and milk production traits in dairy cattle. J Vet Med A Physiol Pathol Clin Med 2000; 47(1): 31–36.
10 10 Mayhew IG. Neuro‐ophthalmology: a review. Equine Vet J Suppl 2010( 37): 80–88.
11 11 Johnson PJ, Constantinescu GM and Frappier BL. The vestibular system. Part I: Anatomy, physiology and clinical signs from altered vestibular function. Equine Vet Educ 2001; 13(2): 105–109.
12 12 Thomson C and Hahn C. Veterinary neuroanatomy: a clinical approach. 1st ed. Saunders Elsevier. 2012; 208.
13 13 Nunnery C, Pickett JP and Zimmerman KL. Congenital stationary night blindness in a Thoroughbred and a Paso Fino. Vet Ophthalmol 2005; 8(6): 415–419.
14 14 Sandmeyer LS, Bellone RR, Archer S, et al. Congenital stationary night blindness is associated with the leopard complex in the Miniature Horse. Vet Ophthalmol 2012; 15(1): 18–22.
15 15 Leiva M, Pena T and Monreal L. Ocular findings in healthy newborn foals according to age. Equine Vet Educ 2011; 23(1): 40–45.
16 16 Jacob SI, Drees R, Pinkerton ME, Bentley EM and Peek SF. Cavernous sinus syndrome in a Holstein bull. Vet Ophthalmol 2015; 18(2): 164–167.
17 17 Myrna KE. Neuro‐ophthalmology in the Horse. Vet Clin North Am Equine Pract 2017; 33(3): 541–549.
18 18 Robillard A, Saint‐Hilaire JM, Mercier M and Bouvier G. The lateralizing and localizing value of adversion in epileptic seizures. Neurology 1983; 33(9): 1241–1242.
19 19 Schiff MM, Esmond WG and Himwich HE. Forced circling movements (adversive syndrome): correction with dinenhydrinate (“dramamine”). Arch Otolaryngol 1950; 51(5): 672–677.
12 Dropped mandible and masticatory muscle atrophy
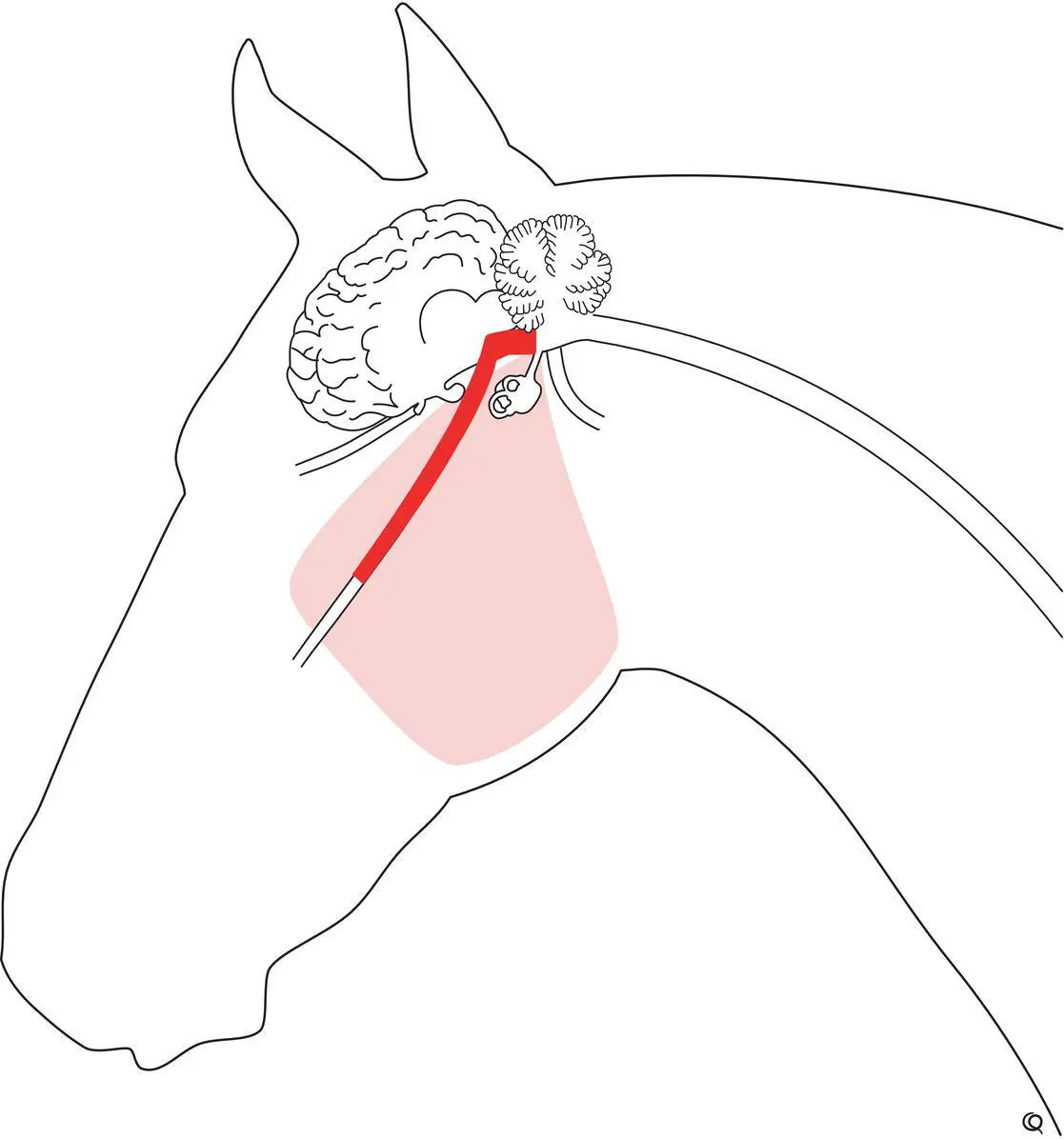
With denervation of the muscles of mastication, the masseter, temporalis, medial and lateral pterygoid, and distal belly of the digastricus muscles on the affected side atrophy rapidly within 1–2 weeks. Unilateral neurogenic muscle atrophy is initially not associated with any major difficulty in chewing food. With time however there is deviation of the mandible toward the normal side ( Figure 12.1).Many normal horses appear to have asymmetric appearance to the origins of the temporal muscles without having asymmetric supraorbital fossae, thus having equal tissue mass behind the eyeballs ( Figure 12.2). On the other hand, some asymmetry of the temporalis and other masticatory muscles occurs quite frequently that is assumed to be associated with dental pain, as it can resolve with adequate dental care. Bilateral atrophy can result in a horse showing ponderous eating habits and dropping food from its mouth. Total mandibular paralysis in all large animals causes the jaw to hang down with food and saliva present in the mouth, and usually the tongue hangs out over the lower incisor teeth.
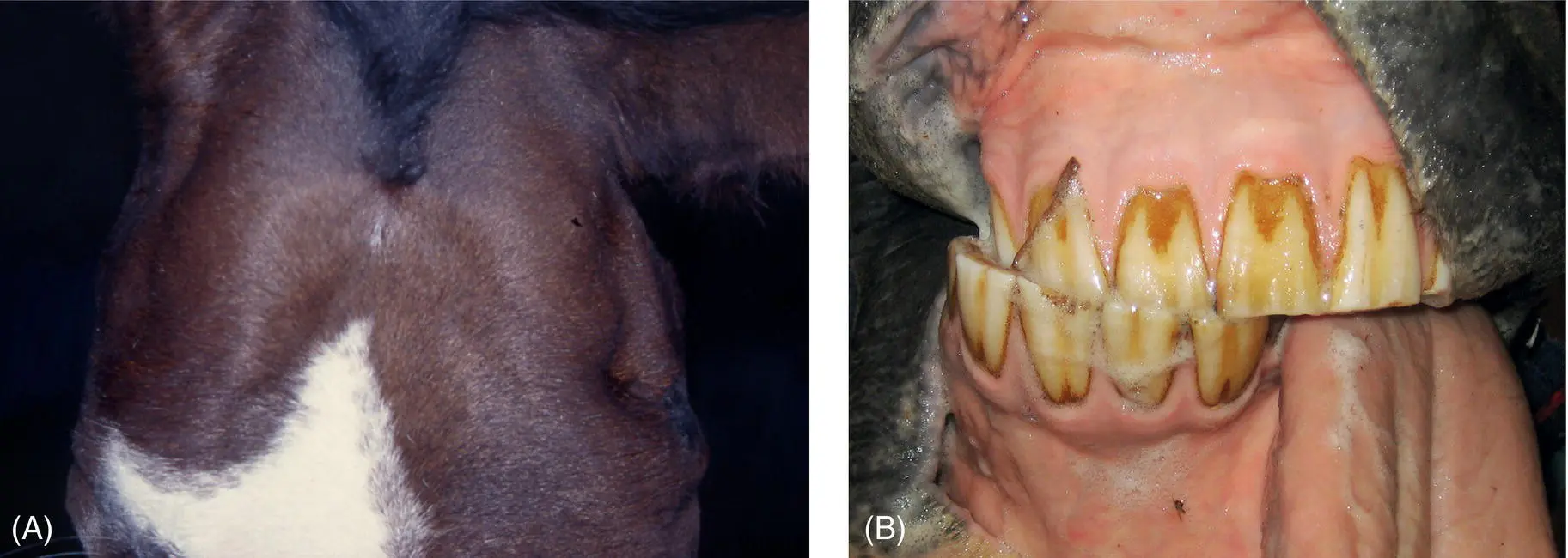
Figure 12.1 This horse suffered total left trigeminal nerve trauma from direct penetrating trauma to the dorsal guttural pouch several months previously. Atrophy of the left muscles of mastication resulted in pronounced sinking of the left supraorbital fossa (A) and the mandible became drawn to the right side with the tongue passively falling from the left side of the mouth (B). The cheek teeth arcades also required dental care.

Figure 12.2 Interpreting asymmetry of muscles of mastication can be problematic. This is especially so for temporalis muscles that often appear asymmetric in normal horses. Both these horses (A and B) have asymmetry to the shape of their parietotemporal regions associated with a difference in the appearance of origin of each pair of temporalis muscles at the temporal crests of the parietal bones (arrows). One good guideline as to whether or not there actually is an asymmetry of muscle bulk between the pairs of temporalis and masseter muscles is whether or not there is an asymmetry to the depth (or filling) of the supraorbital fossae (arrowheads). However, any asymmetry in the bulk of tissue in the bony orbit, to include the eyeballs, muscles, and fat, can result in a change in the appearance of the supraorbital fossae. In actual fact, the first horse (A) had neurogenic atrophy of the temporal muscle associated with S. neurona encephalitis involving the right motor nucleus of the trigeminal nerve. The muscle asymmetry present in the second horse (B) was incidental—very likely due to asymmetric chewing, perhaps resulting from dental disease.
Sudden onset of a dropped mandible in a large animal patient should prompt the clinician to suspect trauma to the temporomandibular joint or mandible, with or without fracture of the mandible ( Figure 12.3). Neurologic diseases resulting in a dropped mandible with no other neurologic signs rarely occur in large animals. An immune‐associated masseter myositis and selective, immune‐associated, transient trigeminal (nongranulomatous) neuritis without systemic polyneuritis do not yet appear to have been documented in large animals. Bilateral myopathy of muscles of mastication occurring in horses, particularly those in poor condition, appears to be related to vitamin E and possibly selenium deficiency. 1This should be differentiated from neurogenic and physical causes of loss of mandibular muscle function. The acute phase of this can result in swollen masseter muscles (palpation of which eliciting a pain response), exophthalmos, chemosis, and protrusion of the third eyelid, 2,3whereas the chronic phase progresses to muscle atrophy, difficulty in eating, and degrees of trismus. 1,4
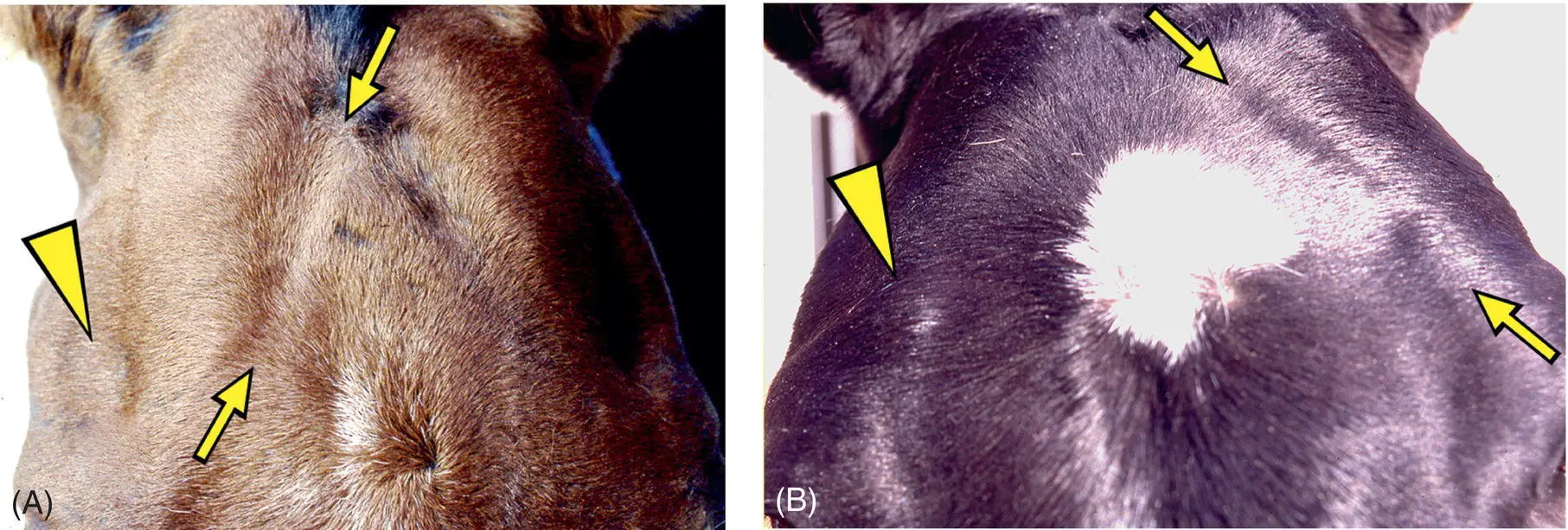
Figure 12.3 Large animals, especially cattle that develop a jaw that does not close, will have the tongue resting slightly out from the mouth (A). That this is not glossal weakness is evident by the patient being able to withdraw the tongue into the mouth with stimulation and by the patient being able to move the tongue to attempt to eat and to lick its nares (B). This calf was involved in a squeeze‐shute accident and lost the ability to close its jaws. No temporomandibular damage could be detected, and as mandibular function returned quite quickly within about a week it was believed that this was due to a motor mandibular nerve neurapraxia, perhaps associated with (sub)luxation of the temporomandibular joint, as the nerve and its branches pass by the joint.
Читать дальше















