Sympathetic denervation of the head in cattle includes the eye signs of ptosis of the upper eyelid, miosis, and subtle enophthalmos as described in other species, with dilated vessels on the pinnae, warm face and ears, and an absence of droplets of sweat forming on the muzzle ( Figure 10.7). Eye signs in Horner syndrome are even less prominent in large animal species other than horses and cattle. 14,15,19Interestingly, it may be reasonable to expect ectoparasites such as ticks to be attracted to skin with altered temperature. 27Facial analgesia was present in a cow that had an ocular squamous cell carcinoma invading the trigeminal nerve. Part of the syndrome included a specific and dense infestation of ticks only on the denervated skin of the face. 27This most probably resulted from cutaneous vasodilation caused by interruption of postganglionic sympathetic fibers distal to those innervating the eyeball that had joined the trigeminal nerve to innervate the skin and blood vessels of the remainder of the face.
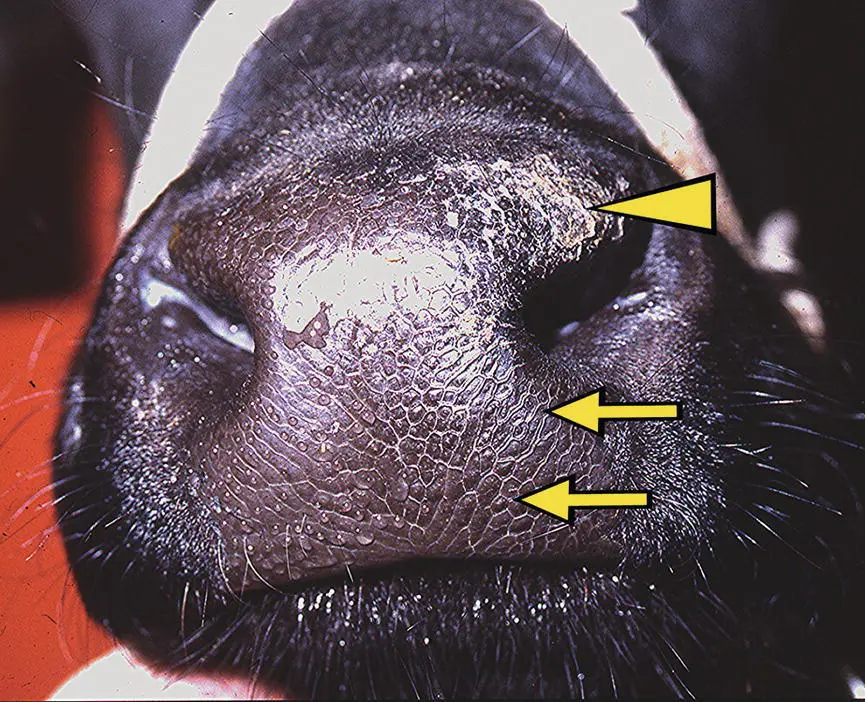
Figure 10.7 Loss of sympathetic control to the blood vessels and glands of the muzzle in cattle results in a loss of fluid production by the nasal glands. This cow had a lesion in the left cranial neck causing Horner syndrome and a dry muzzle on the left side (arrows). Because of the chronicity of the dry muzzle, desiccation and excoriation had begun to occur (arrowhead).
Accompanying generalized signs of autonomic failure, horses affected by equine dysautonomia, commonly known as grass sickness, often show bilateral ptosis. 28, 29Muscles (and their innervation) of the upper eyelid that when paralyzed may result in ptosis of the upper eyelids are the levator palpebrae superioris (CN III), the levator anguli oculi medialis (CN VII), and Müller tarsal smooth muscle (sympathetic). 30In grass sickness cases, there is no evidence for the ptosis being due to somatic facial or oculomotor dysfunction, and the evidence points to this being due to sympathetic dysfunction. Indeed, the ptosis can be readily reversed ( Figure 10.8) using a low dose of topical α‐1 adrenergic agonist (0.5 mL of 0.5% phenylephrine eye drops). Not only does the upper eyelid ptosis resolve within 10–30 min, but the lowered angle of the upper eyelashes ( i.e ., pointing toward the ground), so characteristic of grass sickness, also resolves and often quite impressively so compared to the untreated side. This lower eyelash angle present in grass sickness cases is likely due to paralysis of the smooth muscle innervating the eyelashes themselves, the arrectores ciliorium , present in horses and cattle but not in humans and dogs. This phenylephrine eye drop test is thus useful to assist in the diagnosis of grass sickness and other causes of Horner syndrome at least in horses. 31
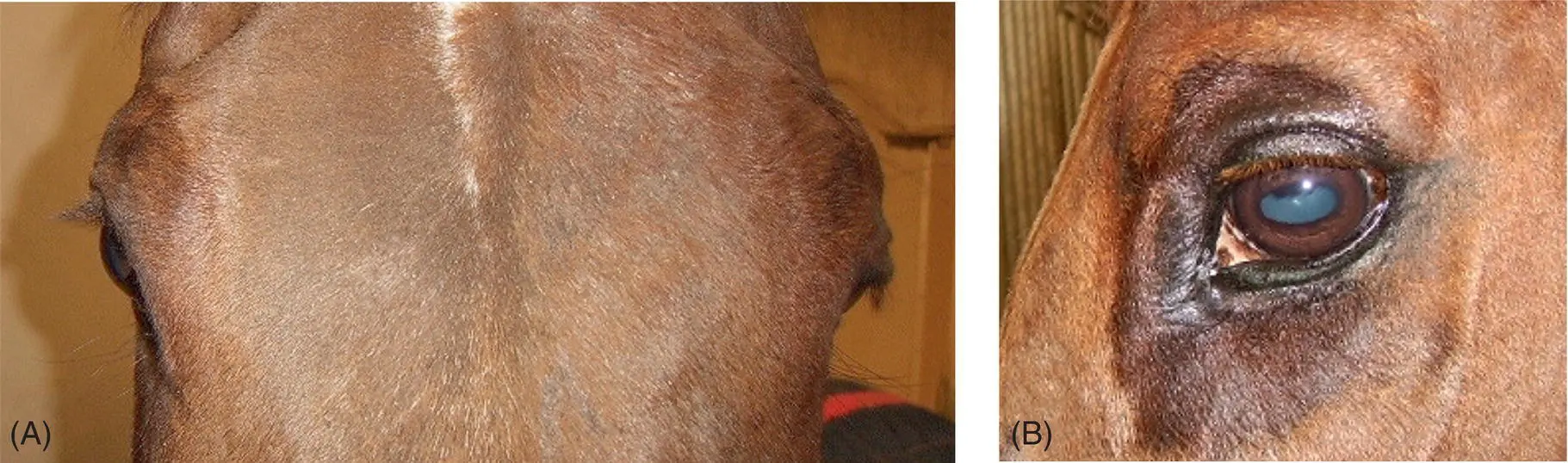
Figure 10.8 This horse has left‐sided Horner syndrome (A). About 20 min following instillation of 0.5 mL of 0.5% phenylephrine into the left conjunctival sac, the ptosis is resolved (B) helping to confirm hypersensitivity to adrenergic compounds in the sympathetically denervated structures. An apparently excessive sweat response to phenylephrine also is seen around the orbit (B).
Third‐order sympathetic neuronal fibers do not pass through the petrosal bone as in small animal species; therefore, Horner syndrome is usually not recognized with otitis media in large animals or with petrosal bone fractures. Inadvertent perijugular injection of drugs is a relatively frequent cause of Horner syndrome when the compound spreads to the adjacent cervical vagosympathetic trunk. The effect with local anesthetic compounds including α‐2 drugs is usually temporary. But depending on the degree of tissue inflammation caused by other, more irritant compounds, any resulting Horner syndrome can last for hours to months and may be permanent. In horses, the sympathetic fibers innervating the eye are more often damaged in and around the guttural pouch in the region of the cranial cervical ganglion. Finally, many systemic toxins, such as those mediated by atropine‐like alkaloids and the common antimuscarinic colic drug butylscopolamine, cause degrees of mydriasis ( Figure 10.1), and those acting with anticholinesterase activity can result in miosis.
1 1 Beltran E, Matiasek K and Hartley C. Equine neuroophthalmology. In Equine Ophthalmology, Gilger BC, Editor. 3rd ed. Wiley‐Blackwell, Oxford, UK. 2016; 567.
2 2 Irby NL. Neuro‐ophthalmology in horses. Vet Clin North Am Equine Pract 2011; 27(3): 455–479.
3 3 Mayhew IG. Neuro‐ophthalmology: a review. Equine Vet J Suppl 2010( 37): 80–88.
4 4 Myrna KE. Neuro‐ophthalmology in the Horse. Vet Clin North Am Equine Pract 2017; 33(3): 541–549.
5 5 Lavach JD. Large Animal Ophthalmology. Mosby, St. Louis, MO. 1990; 395.
6 6 Barnett KC, Crispin SM, Lavach JD and Matthews AG. Equine Ophthalmology: An Atlas and Text. 2nd ed. Saunders, Edinburgh. 2004; 274.
7 7 Park JC, Moura AL, Raza AS, et al. Toward a clinical protocol for assessing rod, cone, and melanopsin contributions to the human pupil response. Invest Ophthalmol Vis Sci 2011; 52(9): 6624–6635.
8 8 Yeh CY, Koehl KL, Harman CD, et al. Assessment of rod, cone, and intrinsically photosensitive retinal ganglion cell contributions to the canine chromatic pupillary response. Invest Ophthalmol Vis Sci 2017; 58(1): 65–78.
9 9 Hall CA and Chilcott RP. Eyeing up the future of the pupillary light reflex in neurodiagnostics. Diagnostics (Basel) 2018; 8(1): 19.
10 10 Kim J, Heo J, Ji D and Kim MS. Quantitative assessment of pupillary light reflex in normal and anesthetized dogs: a preliminary study. J Vet Med Sci 2015; 77(4): 475–478.
11 11 Komaromy AM, Abrams KL, Heckenlively JR, et al. Sudden acquired retinal degeneration syndrome (SARDS): a review and proposed strategies toward a better understanding of pathogenesis, early diagnosis, and therapy. Vet Ophthalmol 2016; 19(4): 319–331.
12 12 Gregoline B. Eponyms. Chapter 15 in AMA Manual of Style: A Guide for Authors and Editors. 11th edition. Oxford University Press. 2020.
13 13 Musk GC, King M and He BL. Horner syndrome in 2 pigs (Sus scrofa) after vascular grafting of the carotid artery and jugular vein. Comp Med 2017; 67(6): 518–523.
14 14 Samimi AS, Shafiian A, Rezaei M, et al. Horner's syndrome and unilateral facial paresis in a goat: a case report. Comp Clin Pathol 2016; 25(2): 469–472.
15 15 Ding P, Tufano R, Campbell‐Malone R, et al. Horner syndrome after carotid sheath surgery in a pig: anatomic study of cervical sympathetic chain. Comp med 2011; 61: 453–456.
16 16 Borges AS and Watanabe MJ. Guttural pouch diseases causing neurologic dysfunction in the horse. Vet Clin North Am Equine Pract 2011; 27(3): 545–572.
17 17 Simoens P, Lauwers H, De Muelenare C, Muylle E and Steenhaut M. Horner's syndrome in the horse: a clinical, experimental and morphological study. Equine Vet J Suppl 1990( 10): 62–65.
18 18 Mayhew IG. Horner's syndrome and lesions involving the sympathetic nervous system. Equine Pract 1980; 2(5): 44–47.
19 19 Smith JS and Mayhew IG. Horner's syndrome in large animals. Cornell Vet 1977; 67: 529–542.
20 20 Purohit RC, McCoy MD and Bergfeld WA, III. Thermographic diagnosis of Horner's syndrome in the horse. Am J Vet Res 1980; 41(8): 1180–1182.
21 21 Palumbo MIP, Moreira JJ, Olivo G, et al. Right‐sided laryngeal hemiplegia and Horner's syndrome in a horse. Equine Vet Educ 2011; 23(9): 448–452.
Читать дальше














