3.2 Thermodynamics of Glasses
Jean‐Luc Garden and Hervé Guillou
CNRS, Institut Néel and Université de Grenoble Alpes, Grenoble, France
Thermodynamics states that the properties of a system in equilibrium depend neither on time nor on past history. Glasses clearly violate this postulate. Not only do their properties depend on history but they also vary with time at temperatures at which relaxation toward internal thermodynamic equilibrium does occur, but at a rate slow enough to be observable at the timescale of the experiment performed. To deal with glasses, thermodynamics must thus consider nonequilibrium states and their actual cause, namely the irreversibility of the transition that occurs when relaxation times eventually become much longer than experimental timescales such that the material freezes in as a glass.
Much attention is currently paid to the processes driving the glass transition at a microscopic scale and also to their implications for the macroscopic properties of glasses. Because this topic is extensively discussed in this chapter, we will deal here with a second fundamental issue, namely that of the phenomenological approaches followed to understand the observable macroscopic properties of glasses and, thus, to design new applications. To quote a single example, density gradients in tempered glasses are the key to thermal strengthening, which is achieved irreversibly upon cooling ( Chapter 3.12).
In this chapter, the basic concepts of macroscopic nonequilibrium thermodynamics will first be summarized and illustrated with experimental heat capacities for a model system, PolyVinylAcetate [PVAc, (C 4H 6O 2) n]). The basic concepts of equilibrium and nonequilibrium will then be introduced to point out why glasses challenge the laws of thermodynamics. Next, properties of the supercooled liquid state above T gwill be presented and the phenomenology of the glass transition examined in the light of calorimetric data, in particular in terms of configurational properties. The basics of nonequilibrium thermodynamics in the glass transition range will finally be reviewed along with the issue of aging below the glass transition range.
2 Basics of Nonequilibrium Thermodynamics
In thermodynamics one investigates the changes occurring when a system passes from a state A to another state B. At constant chemical composition, the system is in internal equilibrium if its state is defined by only two macroscopic variables such as temperature ( T ), pressure ( P ), volume ( V ), enthalpy ( H ), internal energy ( U ), or Gibbs free energy ( G ). Their values are not only constant but independent of the pathway actually followed between any two states A and B. As stated by the First Law of thermodynamics, between A and B the internal energy varies as:
(1) 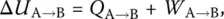
where Q A → Band W A → Bare the heat and work exchanged by the system with its surroundings, respectively. Likewise, the entropy is decomposed into two parts,
(2) 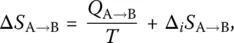
where the first represents the heat exchanged with the surroundings and the second the entropy created within the system itself during the transformation.
If two equilibrium states are connected by a reversible process, then Δ i S A → B= 0. If the system undergoes instead an irreversible process through which it falls out of equilibrium, then Δ i S A → B> 0 since a spontaneous process is always associated with an entropy increase of the system. Upon glass formation by cooling, pressure increase, or other means, the equilibrium liquid is continuously losing internal equilibrium. As will be discussed here, the question arises as to whether there is any finite production of entropy and – if so – whether this quantity is of importance regarding the other terms involved in the process.
The Third Law of thermodynamics postulates that the entropy of a perfectly ordered system is zero at 0 K. In contradiction with it, however, calorimetric measurements indicate that glasses not only possess nonzero entropies at 0 K but that this residual entropy depends on thermal history as illustrated by a simple entropy cycle calculated from measured heat‐capacities and entropy of fusion (Δ S f). Beginning with a perfectly ordered crystal, whose entropy thus is 0 at 0 K, one derives the entropy of the crystal at its congruent temperature of fusion T f, then that of the melt from this temperature down to the glass transition, and finally that of the glass down to 0 K ( Figure 1). The difference S 0between this entropy and that of the crystal at 0 K is the residual entropy ( Table 2), which increases with higher glass transition temperatures and, thus, with higher cooling rates, reflecting the increasingly wide distribution of configurational states obtaining with increasing temperatures.
A finite residual entropy at 0 K might seem to contradict the Third Law of thermodynamics. As justified by Jones and Simon [1], however, there is no contradiction because this law applies only to crystals and other systems in internal equilibrium, which are necessarily ordered at 0 K to minimize their Gibbs free energies. This is not the case of glasses, which do obey the Nernst theorem [2] since they cannot pass from one entropy state to another at 0 K (Δ S = 0 for two neighboring glassy states at 0 K).
Although such determinations also made for partially disordered crystals like ice I hor CO have long been explained by simple statistical mechanical models, the very concept of residual entropy has recently been debated [3]. On the assumption that ergodicity must hold for the entropy to be defined, the proponents of a kinetic view have claimed that the configurational entropy undergoes an abrupt jump at the glass transition in order to reach the zero value of the crystal entropy at 0 K. In contrast, the proponents of the conventional view have stressed that what matters is not time averages but spatial averages of configurational microstates [3], which is the reason why the measured residual entropies do make sense physically and correlate with the specific structural features of glasses and disordered crystals.
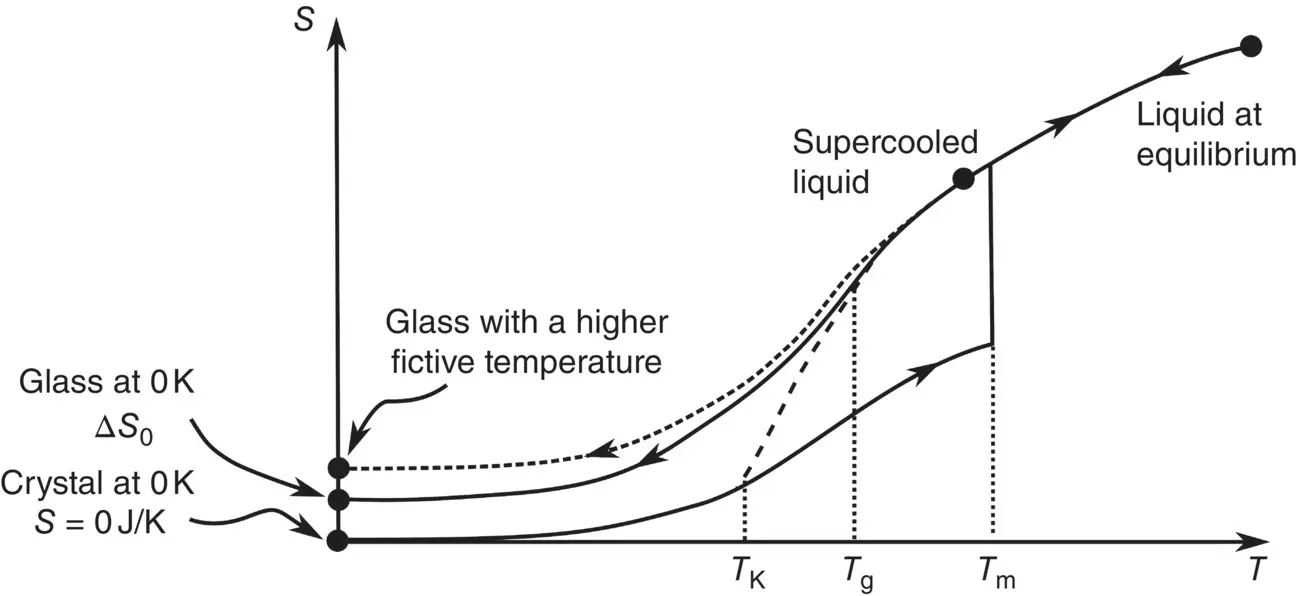
Figure 1 Entropies of the crystal, liquid, supercooled liquid and glass phases of a substance.
By definition, equilibrium thermodynamics cannot alone account for fundamental questions raised when relaxation is too slow with respect to experimental timescales. Owing to the kinetic nature of the problem, use has been made of the formalism originally developed for the kinetics of chemical reactions by De Donder and his school [4]. With values increasing as the reaction proceeds, a new variable, the advancement of reaction, ξ ( t ) is defined to characterize the state of the system as a function of time, t , such that the reaction rate is simply d ξ ( t )/d t . This extensive variable, expressed in mol, accounts for the distribution of matter (local mass or density variation), or the molecular structure, within the system at any time. A new state function, the affinity, A , is then introduced to relate ξ ( t ) to the driving force of the reaction, its Gibbs free energy (at constant T and P ):
Читать дальше