Encyclopedia of Glass Science, Technology, History, and Culture
Здесь есть возможность читать онлайн «Encyclopedia of Glass Science, Technology, History, and Culture» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Encyclopedia of Glass Science, Technology, History, and Culture
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:3 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
Encyclopedia of Glass Science, Technology, History, and Culture: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Encyclopedia of Glass Science, Technology, History, and Culture»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
The
has been designed to satisfy the needs and curiosity of a broad audience interested in the most varied aspects of material that is as old as the universe. As described in over 100 chapters and illustrated with 1100 figures, the practical importance of glass has increased over the ages since it was first man-made four millennia ago. The old-age glass vessels and window and stained glass now coexist with new high-tech products that include for example optical fibers, thin films, metallic, bioactive and hybrid organic-inorganic glasses, amorphous ices or all-solid-state batteries.
In the form of scholarly introductions, the Encyclopedia chapters have been written by 151 noted experts working in 23 countries. They present at a consistent level and in a self-consistent manner these industrial, technological, scientific, historical and cultural aspects. Addressing the most recent fundamental advances in glass science and technology, as well as rapidly developing topics such as extra-terrestrial or biogenic glasses, this important guide:
Begins with industrial glassmaking Turns to glass structure and to physical, transport and chemical properties Deals with interactions with light, inorganic glass families and organically related glasses Considers a variety of environmental and energy issues And concludes with a long section on the history of glass as a material from Prehistory to modern glass science The
has been written not only for glass scientists and engineers in academia and industry, but also for material scientists as well as for art and industry historians. It represents a must-have, comprehensive guide to the myriad aspects this truly outstanding state of matter.

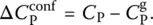
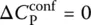 . In this state, only the vibrational motions, i.e. the fast degrees of freedom (faster than the experimental timescale), contribute. To define this contribution over the entire temperature interval of interest, an extrapolation of the glass heat capacity from low to high temperatures is needed ( Figure 2). The heat capacity of the supercooled liquid can also be extrapolated toward low temperatures ( Figure 2). The difference between these values for the supercooled liquid and the glass,
. In this state, only the vibrational motions, i.e. the fast degrees of freedom (faster than the experimental timescale), contribute. To define this contribution over the entire temperature interval of interest, an extrapolation of the glass heat capacity from low to high temperatures is needed ( Figure 2). The heat capacity of the supercooled liquid can also be extrapolated toward low temperatures ( Figure 2). The difference between these values for the supercooled liquid and the glass,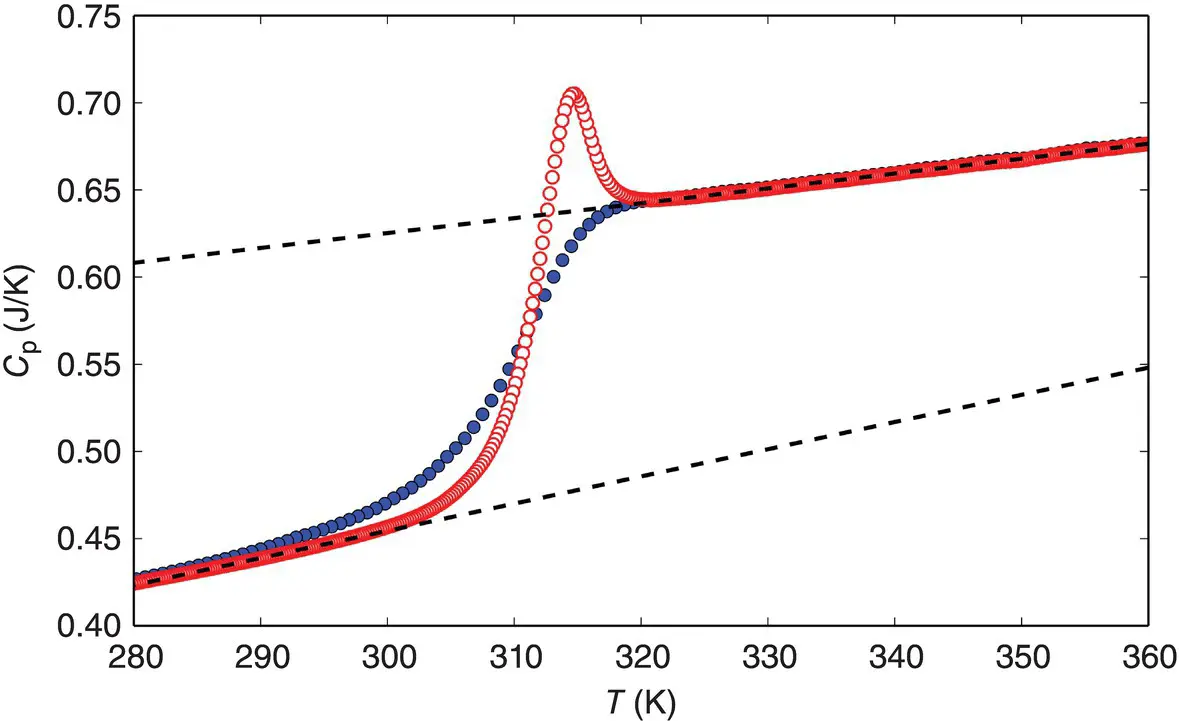
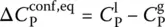

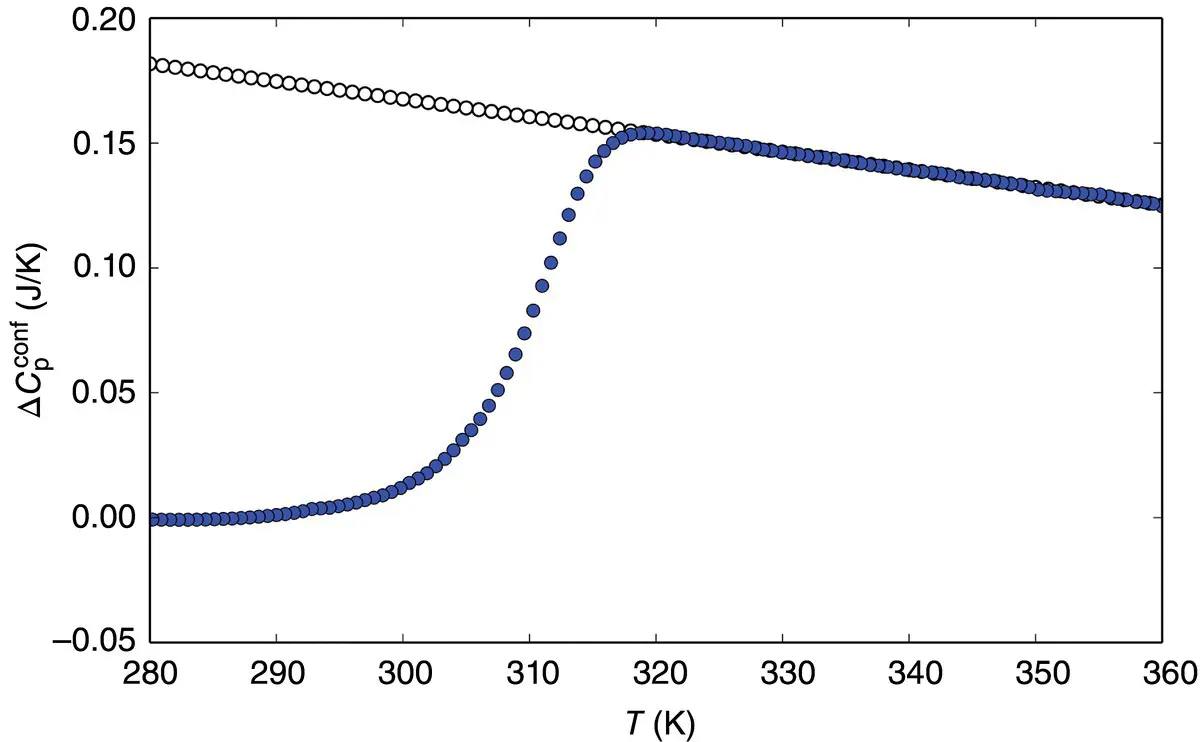
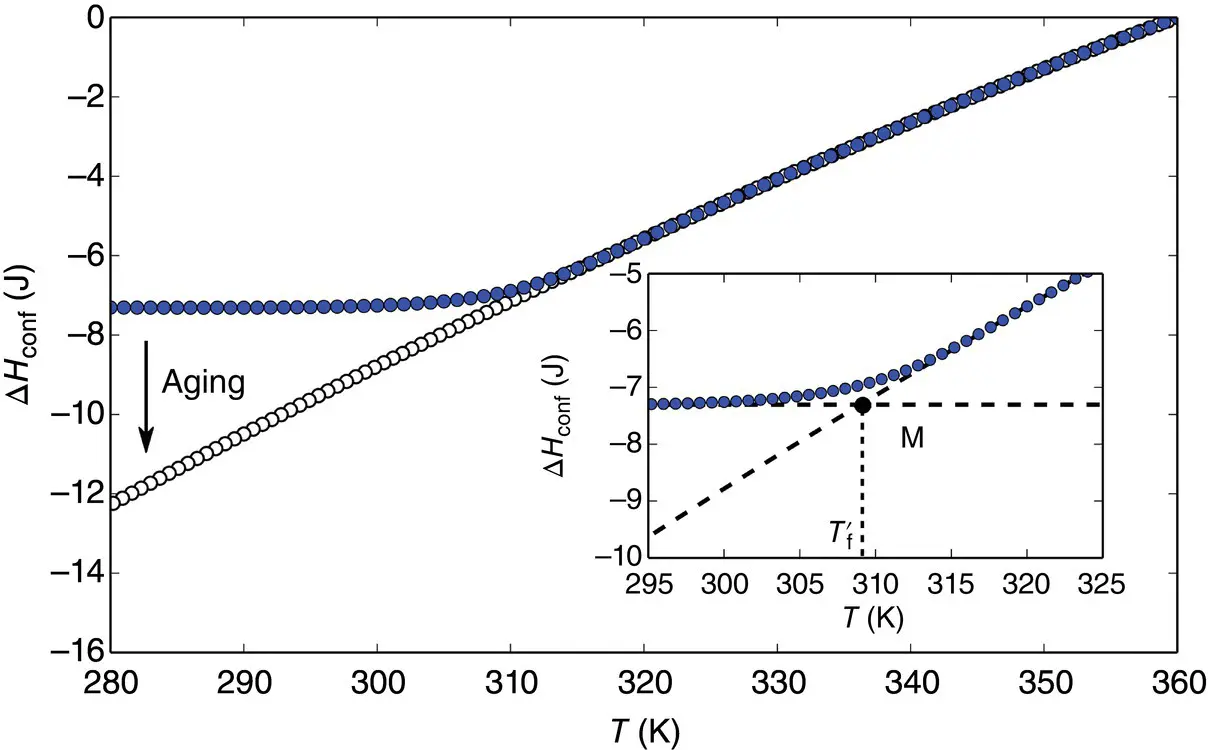
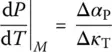
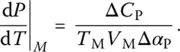
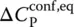 of Eq. (7), Prigogine and Defay [7] assumed that the supercooled liquid is in internal equilibrium down to T M(i.e. A = 0 and d A = 0) whereas the glass below T Mis defined by d ξ = 0. These two equalities can then be grouped to yield the so‐called Prigogine–Defay (PD) ratio [7]:
of Eq. (7), Prigogine and Defay [7] assumed that the supercooled liquid is in internal equilibrium down to T M(i.e. A = 0 and d A = 0) whereas the glass below T Mis defined by d ξ = 0. These two equalities can then be grouped to yield the so‐called Prigogine–Defay (PD) ratio [7]: