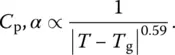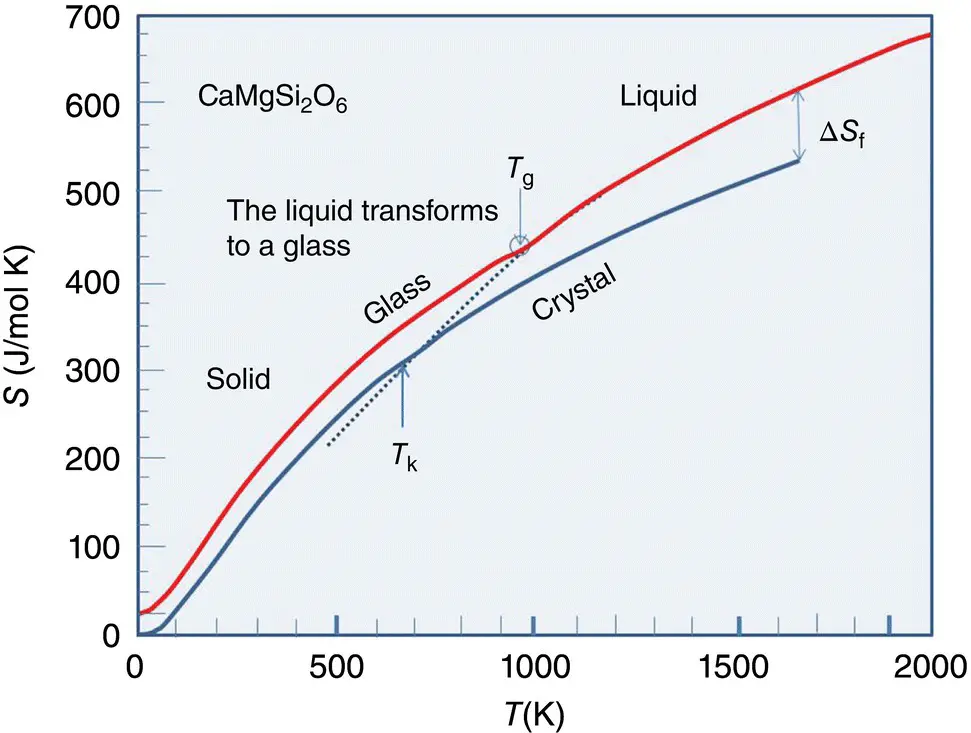
Figure 4 Entropy of the amorphous and crystalline phases of diopside, CaMgSi 2O 6.
Source: After [8].
The liquid transforms into a glass below T g, therefore the entropy of condensed phase (upper curve) does not follow the dashed line which is an extension of liquid entropy curve below T g.
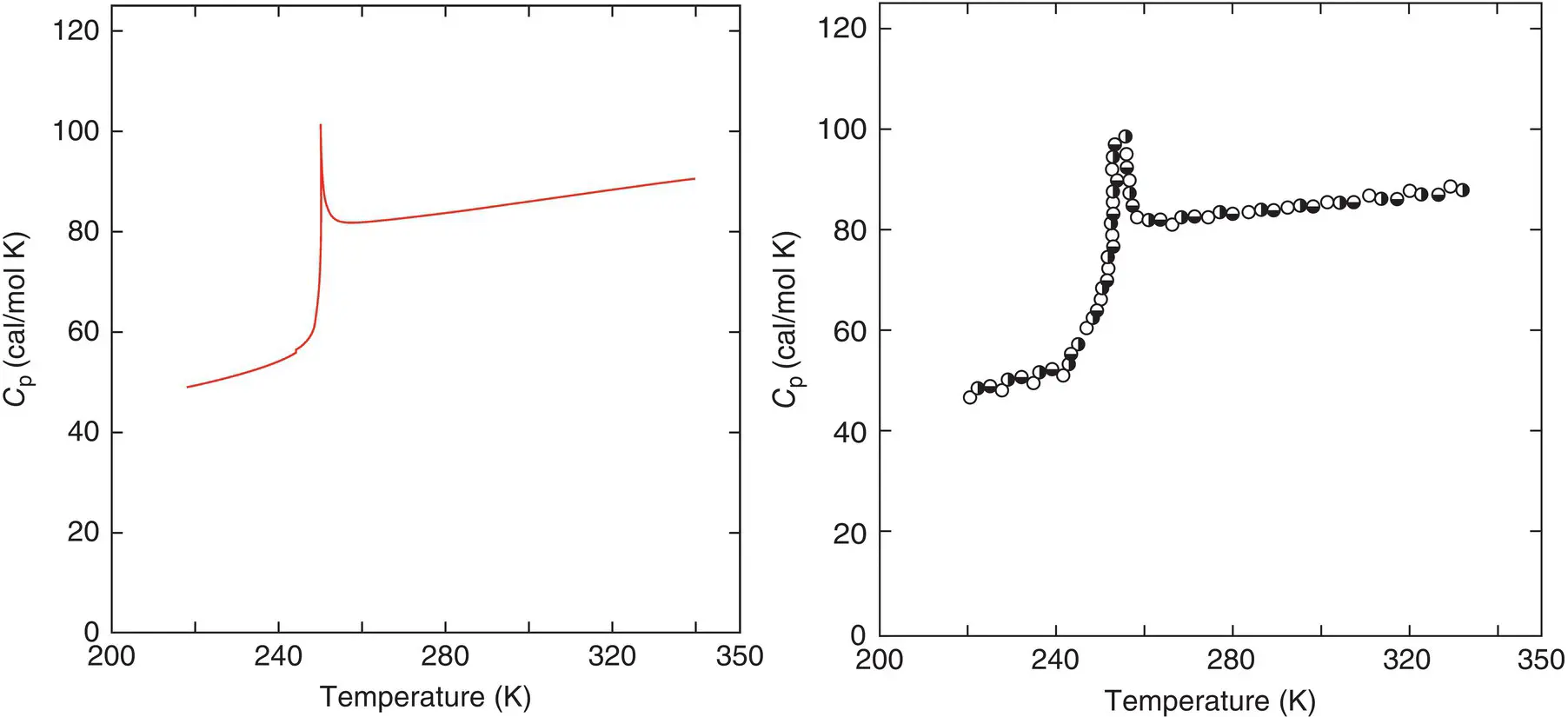
Figure 5 Comparison between the heat capacities of amorphous o ‐terphenol measured and calculated with configuron percolation theory.
Source: After [3].
(12) 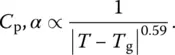
A last feature deserving to be mentioned is the “universal” dependence of the light scattering intensity on the time after a temperature jump in the glass transition range of oxide glasses, which is known as the Bokov effect [33]. The intensity displays a maximum whose height and location on the timescale depends on the previous history of the glass. The Bokov effect is associated with nonequilibrium fluctuations produced by coupling between hydrodynamic modes. Detailed investigations in the past decade have demonstrated that similarities observed in the glass transition region of oxides and polymers account for structural transformations related to the formation of spatially extensive structures, which in turn could be related to clustering effects similar to that envisaged by CPT and other similar models. The Bokov effect thus is providing additional arguments to characterize the glass transition as a second order like phase transformation rather than simply as a slowing down of dynamic processes.
Understanding vitrification mechanisms is of great importance either practically or theoretically. Although progress made in this respect has been very impressive, many of the questions remain unresolved. Among them, a central one is that of the glass transition itself, which has a pronounced relaxational, kinetic character in spite of its similarity with a second‐order phase transition in the Ehrenfest sense with volume and entropy continuity, but discontinuities of their derivatives that are used in practice to detect T g. Discussion about the nature of glass continues. After some lull it has gathered new momentum, especially in the second decade of the new century as the microscopic mechanisms generating the glassy state of matter are still debated. Future developments could be based on computer modeling that does also show the appearance of discontinuities in derivative thermodynamic parameters at the glass transition.
The author acknowledges help and advice from R. Doremus, V.L. Stolyarova, P. Poluektov, E. Manykin, W.E. Lee, P. James, R.J. Hand, K.P. Travis, G. Moebus, J.M. Parker, A. Varshneya, O.V. Mazurin, M. Liska, J. Marra, C.M. Jantzen, R. Tournier, C.A. Angell, and D.S. Sanditov.
1 1 Zarzycki, J. (1982). Glasses and the Vitreous State. Cambridge: Cambridge University Press.
2 2 McNaught, A.D. and Wilkinson, A. (eds.) (1997). The IUPAC Compendium on Chemical Terminology. Cambridge: Royal Society of Chemistry.
3 3 Ojovan, M.I. and Lee, W.E. (2006). Topologically disordered systems at the glass transition. J. Phys. Condens. Matter 18: 11507–11520.
4 4 Schairer, J.F. and Bowen, N.L. (1956). The system Na2O‐Al2O3‐SiO2. Am. J. Sci. 254: 129–195.
5 5 Tangeman, J.A., Phillips, B.L., Navrotsky, A. et al. (2001). Vitreous forsterite (Mg2SiO4): synthesis, structure, and thermochemistry. Geophys. Res. Lett. 28: 2517–2520.
6 6 Richet, P., Roskosz, M., and Roux, J. (2006). Glass formation in silicates: insights from composition. Chem. Geol. 225: 388–401.
7 7 Sakka, S., Sakaino, T., and Takahashi, K. (eds.) (1975). Glass Handbook. Tokyo: Asakura Publishing Co.
8 8 Mysen, B.O. and Richet, P. (2005). Silicate Glasses and Melts. Properties and Structure. Amsterdam: Elsevier.
9 9 Varshneya, A.K. (2006). Fundamentals of Inorganic Glasses. Sheffield: Society of Glass Technology.
10 10 Uhlmann, D.R. (1972). A kinetic treatment of glass formation. J. Non Cryst. Solids 7: 337–348.
11 11 Cohen, M.H. and Turnbull, D. (1961). Composition requirements for glass formation in metallic and ionic systems. Nature 189: 131–132.
12 12 Doremus, R.H. (2003). Melt viscosities of silicate glasses. Am. Ceram. Soc. Bull. 82: 59–63.
13 13 Volf, M.B. (1988). Mathematical Approach to Glass. Amsterdam: Elsevier.
14 14 Ojovan, M.I. (2012). Viscous flow and the viscosity of melts and glasses. Phys. Chem. Glasses 53: 143–150.
15 15 Zheng, Q. and Mauro, J.C. (2017). Viscosity of glass‐forming systems. J. Am. Ceram. Soc. 100: 6–25.
16 16 Angell, C.A. and Rao, K.J. (1972). Configurational excitations in condensed matter, and the “bond lattice” model for the liquid‐glass transition. J. Chem. Phys. 57: 470–481.
17 17 Ojovan, M.I., Travis, K.P., and Hand, R.J. (2007). Thermodynamic parameters of bonds in glassy materials from viscosity temperature relationships. J. Phys. Condens. Matter 19: 415107.
18 18 Zachariasen, W.H. (1932). The atomic arrangement in glass. J. Am. Chem. Soc. 54: 3841–3851.
19 19 Smekal, A. (1951). On the structure of glass. J. Soc. Glass Technol. 35: 411–420.
20 20 Stanworth, J. (1952). Tellurite glasses. J. Soc. Glass Technol. 36: 217–241.
21 21 Sun, K.‐H. (1947). Fundamental condition of glass formation. J. Am. Ceram. Soc. 30: 277–281.
22 22 Rawson, H. (1967). Inorganic Glass‐Forming Systems. London: Academic Press.
23 23 Boubata, N., Roula, A., and Moussaoui, I. (2013). Thermodynamic and relative approach to compute glass‐forming ability of oxides. Bull. Mater. Sci. 36: 457–460.
24 24 Dietzel, A. (1948). Glasstruktur und Glaseigeschaften. Glastech. Ber. 22: 41–50.
25 25 Vogel, W. (1994). Glass Chemistry, 2e. New York: Springer.
26 26 Phillips, J.C. (1979). Topology of covalent non‐crystalline solids I. J. Non Cryst. Solids 34: 153–181.
27 27 Ojovan, M.I. (2013). Ordering and structural changes at the glass‐liquid transition. J. Non Cryst. Solids 382: 79–86.
28 28 Stolyarova, V.L. (2008). Thermodynamic properties and structure of ternary silicate glass‐forming melts: experimental studies and modelling. J. Non Cryst. Solids 354: 1373–1377.
29 29 Mandelbrot, B.B. (1982). The Fractal Geometry of Nature. San Francisco: W. H. Freeman and Co., 460pp.
30 30 Mazurin, O.V. and Gankin, Y.V. (2008). Glass transition temperature: problems of measurement procedure. Glass Technol. 49: 229–233.
31 31 Sanditov, D.S. and Ojovan, M.I. (2017). On relaxation nature of glass transition in amorphous materials. Physica B 523: 96–113.
32 32 Kauzmann, W. (1948). The nature of the glassy state and the behaviour of liquids at low temperatures. Chem. Rev. 43: 219–256.
33 33 Bokov, N.A. (2008). Non‐equilibrium fluctuations as a plausible reason of the light scattering intensity peak in the glass transition region. J. Non Cryst. Solids 354: 1119–1122.
1 Reviewers:R. Hand, Department of Materials Science and Engineering, University of Sheffield, Sheffield, UKV. Stolyarova, Saint Petersburg State University, Saint Petersburg, Russian Federation
Читать дальше