The strong points of Zachariasen's model are that it predicts the existence of the main oxide glass formers (SiO 2, GeO 2, B 2O 3, P 2O 5, etc.) and glass modifiers (Na 2O, CaO, etc.) and makes room for the distinction between bridging (BO) and non‐bridging oxygens (NBO). Its main limitation is that it does not consider at all modified oxides or multicomponent systems, or even non‐oxide glasses. In addition, several exceptions to its rules are found as exemplified by alumina‐lime glasses and chain‐like glass structures (e.g. metaphosphate glasses).
Smekal [19] thus developed the concept of the mixing bond nature of (good) glass formers. He noted that pure covalent bonds are incompatible with a random arrangement in view of sharply defined bond lengths and angles. Purely ionic or metallic bonds lack any directional characteristics so that the presence of mixed chemical bonding is necessary for glass formation. Indeed, known glass formers obey this concept: (i) inorganic compounds like SiO 2or B 2O 3where the A─O bonds are partly covalent and partly ionic; (ii) elements (S, Se) having chain structures with covalent bonds within the chain and van der Waal's forces between them ( Chapter 6.5); and (iii) organic compounds containing large molecules with covalent internal bonds and van der Waals' forces between the molecules ( Chapter 8.8).
Alternatively, Stanworth proposed a criterion for glass formation according to which the electronegativity of cations in oxide glasses falls within a certain range between 1.90 and 2.20 [20]. Although the electronegativity values of the constituent atoms can be used to predict the formation of many glasses, this criterion cannot account for systems when bond strength needs to be considered as a secondary criterion.
Sun developed the bond‐strength model on the assumption that, when a melt vitrifies, the stronger the metal–oxygen bond, the more difficult the structural rearrangements necessary for crystallization become and, hence, the easier is glass formation. Glass formation is then ensured by the connectivity of bridging bonds combined with strong bonding between atoms (ions) [21]. Sun thus classified oxides according to their bond strengths so that glass formers form strong bonds with oxygen to yield a rigid network, which results in a high viscosity. He defined modifiers as weakly bonding with oxygen in such a way that they disrupt and modify the network. Without producing glasses on their own, they do form intermediate bonds with oxygen and thus aid vitrification with other oxides.
In practical terms, Sun's energy criterion establishes a correlation between the glass‐forming tendency and the strength of the bond between the elements and the anion in the glass. The single bond strength E bwas defined as:
(8) 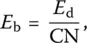
where CN is the coordination number and E dthe dissociation energy of oxides into their gaseous elements. For B 2O 3, SiO 2, GeO 2, P 2O 5, or Al 2O 5, the single bond strength of network formers with oxygen is higher than 330 kJ/mol. Values lower than 250 kJ/mol hold for network modifiers such as Li, Na, K, Mg, or Ca whereas intermediate cations such as Ti, Zn, and Pb are characterized by values intermediate between these two figures.
For both Zachariasen and Sun models Al +3is a challenge. Although Al 2O 3satisfies Zachariasen's criteria, it does not form a glass. Likewise, with E d= 1320–1680 kJ, alumina should be a glass former at a CN of 4 for Al 3+and a modifier at a CN of 6. Sun model was also specific to oxides. It did not account for chalcogenide glasses ( Chapter 6.5) with typical bond strength of 170 kJ/mol along the chains (covalent bond) and less between the chains (van der Waals forces). An interesting intermediate class of oxide is formed by TeO 2, MoO 3, Bi 2O 3, Al 2O 3, Ga 2O 3, and V 2O 5, which do not vitrify by themselves but will do so when mixed with other (modifier) oxides.
Rawson modified Sun's criteria for glass formation [22] by considering the ratio of the bond strength and energy available at the melting point T minstead of the coordination number. He noted that glass formation then correlates better with E b/ T m, being achieved for values of this ratio higher than 0.05 kJ/mol K. The higher this value, the lower the probability for bonds to be broken at T m, and hence the greater the vitrifiability. Glass formation is thus easier for high bond strength and low melting (liquidus) temperature, which implies that eutectic compositions do favor it.
Based on Rawson and Sun approaches, a modified glass‐forming criterion termed the Thermodynamic Relative Glass‐Forming Ability has recently been proposed in terms of the parameter E b/ C p T m,where C pis the isobaric heat capacity [23], which can be regarded as an extension of Rawson's criteria. The ordering that results from this model is not convincing, however, and its basic ideas remain to be well justified.
Dietzel [24] characterized the ability of cations to enter the network structure by their field strength, which he defined as
(9) 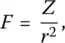
where Z is its valence and r its ionic radius (Å) in the oxide. As listed in Table 2, lower field‐strength cations (e.g. alkalis) are network modifiers, whereas ions with higher field strengths (such as Si, P, or B) are network formers. Interestingly, Dietzel model is appropriate for describing phase separation, either through crystallization or unmixing, in cooled binary systems such as SiO 2–P 2O 5, SiO 2–B 2O 3, or B 2O 3–P 2O 5. This may generally be the case when the field strengths of two cations are approximately equal since forming a single stable crystalline compound normally requires a difference Δ F greater than 0.3. The number of possible stable compounds increases with Δ F as well as the tendency to form glass. For a binary system, glass formation is likely for Δ f larger than 1.33 although this theory is useful to categorize the glass‐forming ability of conventional systems, but not universally [25].
Finally, the topological constraints theory introduced by Phillips [26] must also be mentioned. As reviewed in Chapter 2.7, it indicates that the glass‐forming tendency is maximized when the number of constraints is equal to the number of degrees of freedom in the structure.
In summary, vitrification is favored by high viscosity and configurational complexity. A more complicated chemical composition translates into a greater number of compounds that could nucleate. Owing to mutual competition between these possible crystals, nucleation and growth crystals end up being frustrated. That they do not take place upon rapid cooling thus is a consequence of a confusion principle [8].
6 Glass‐Liquid Transition
Structural theories with energetic and microstructural criteria such as topological constraints describe elements that favor glass formation, i.e. the preservation of a topologically disordered distribution of basic elements in glasses. Kinetic theory shows how to avoid crystallization rather than explaining why the vitreous state really forms through the liquid–glass transition – it is at T gthat the “drama” occurs! Although kinetically controlled, the glass transition manifests itself as a second‐order phase transformation in the sense of Ehrenfest classification. Depending on the kind of measurement performed, it is thus revealed either as a continuous change of first‐order thermodynamic properties such as volume, enthalpy, entropy, or as a discontinuous variation of second‐order thermodynamic properties such as heat capacity or thermal expansion coefficient across the glass transition range.
Читать дальше